- Our Services
- Platforms
- Target Solutions
- Technologies
- Service Types
- Our Science
- About Us
- Contact us
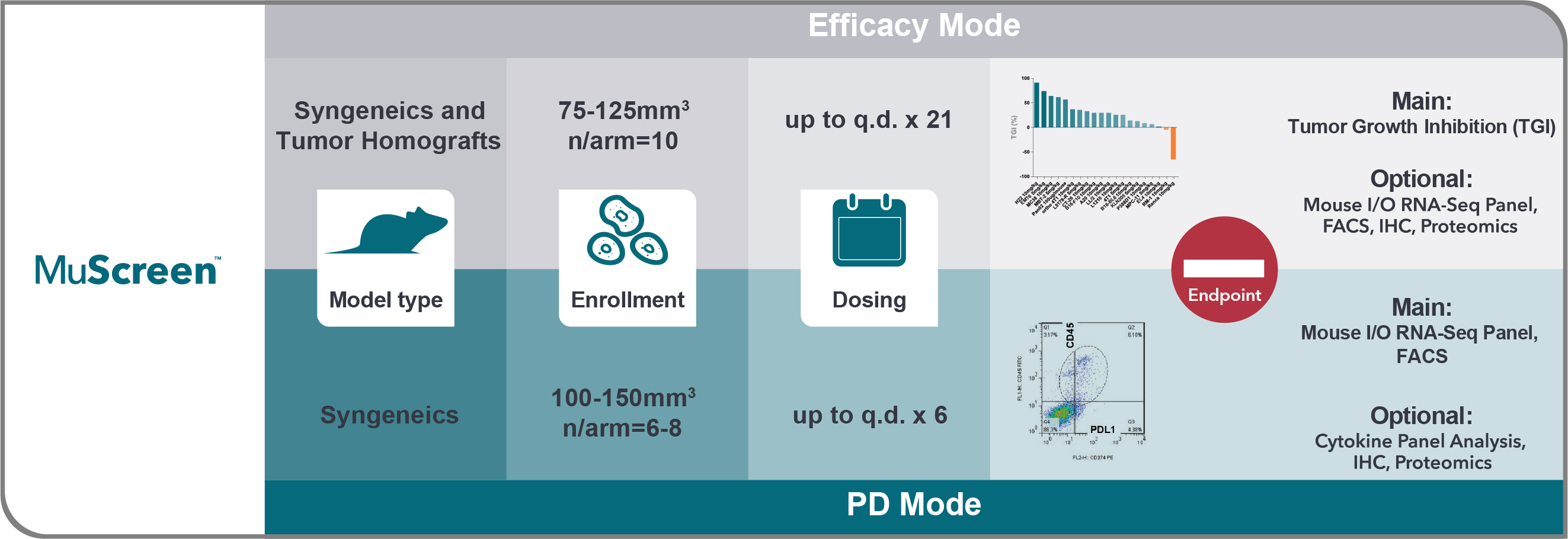
The MuScreen platform is the first high throughput in vivo screen to evaluate immune modulating agents across preselected panels of well-characterized syngeneic and tumor homograft (MuPrime™) models.
This screen provides a cost effective and time efficient way to fast-track in vivo preclinical immunotherapy development.

Choose MuScreen to:
| Model Type | Model Features | Efficacy | PD | ||
|---|---|---|---|---|---|
| US | China | US | China | ||
| Syngeneic | Display immune heterogeneity and diversity observed in the clinic |
6 models |
12 models |
6 models |
12 models |
| Tumor Homograft | Transplants of GEMM tumors into a syngeneic host, preserving original GEMM tumor molecular and histopathology and clinically relevant oncogenic driver mutations |
6 models |
|||
| Registration Deadline | Study Initiation |
|---|---|
| January 1, 2025 | February 21, 2025 |
| April 18, 2025 | May 16, 2025 |
| July 18, 2025 | August 15, 2025 |
| September 19, 2025 | October 17, 2025 |
| Registration Deadline | Study Initiation |
|---|---|
| January 24, 2025 | February 21, 2025 |
| April 18, 2025 | May 16, 2025 |
| July 18, 2025 | August 15, 2025 |
| September 19, 2025 | October 17, 2025 |
| Registration Deadline | Study Initiation |
|---|---|
| January 10, 2025 | February 21, 2025 |
| May 9, 2025 | June 20, 2025 |
| September 5, 2025 | October 17, 2025 |
Choose between 6 or 12 well-characterized syngeneic models to evaluate your I/O compound efficacy or PD effect.
| Cancer Type | Model | Mouse Strain | Immune Cell Profiling | RNAseq | Baseline Proteomics Data |
|---|---|---|---|---|---|
| Breast | EMT6* | BALB/c | Yes | Yes | Yes |
| Bladder | MB49 | C57BL/6 | Yes | Yes | No |
| Colorectal | CT26.WT* | BALB/c | Yes | Yes | Yes |
| MC38 | C57BL/6 | Yes | Yes | Yes | |
| Kidney | Renca | BALB/c | Yes | Yes | Yes |
| Liver | H22^ | BALB/c | Yes | Yes | Yes |
| Hepa 1-6 | C57BL/6 | Yes | Yes | Yes | |
| Lung | LL/2 | C57BL/6 | Yes | Yes | Yes |
| Lymphoma | A20* | BALB/c | Yes | Yes | Yes |
| Melanoma | B16-F10* | C57BL/6 | Yes | Yes | Yes |
| Pancreatic | Pan02* | C57BL/6 | Yes | Yes | Yes |
| Prostate | RM-1* | C57BL/6 | Yes | Yes | Yes |
* Models are run at our US site in a 6 model MuScreen
^ This line is not applicable for FACS
Syngeneic models display immune heterogeneity and diversity observed in the clinic, enabling comprehensive evaluation of efficacy and PD effects of your immune modulating agents.
Key advantages of syngeneic models include:
Our syngeneic models are highly characterized, with data including:
Visit MuBase® for all available model data
Test your I/O agent antitumor efficacy in vivo on a panel of our unique tumor homograft models
| Cancer Type | Model | Mutations/Carcinogen | Strain Background |
Immune Profiling |
RNAseq | Growth Curve |
SoC Data |
|---|---|---|---|---|---|---|---|
| Breast | mBR6004 | MMTV-PyVT TG | FVB/N | Yes | Yes | Yes | Yes |
| Liver | mLI9040 | Alb-Cre; CAG-LSL-cMyc | C57BL/6 | Ongoing | Yes | Yes | Yes |
| Lung | mLU6045 | Kras(G12D); P53-/- | C57BL/6 | Yes | Yes | Yes | Yes |
| Pancreatic | mPA6115 | Kras(G12D); P53-/-; PDx-1 cre | C57BL/6 | Yes | Yes | Yes | Yes |
| Sarcoma | mSA9003 | P53-/- | C57BL/6 | Yes | Yes | Yes | Yes |
| Skin | mSK6005 | ApcMin/+ | C57BL/6 | Yes | Yes | Yes | Yes |
Tumor homografts are transplants of spontaneous or carcinogen-induced GEMM tumors in immunocompetent syngeneic hosts. They preserve the original GEMM tumor molecular and histopathology as well as clinically relevant oncogenic driver mutations.
Our MuPrime tumor homografts have been developed to broaden the number and molecular pathology of preclinical syngeneic models for in vivo pharmacology studies.
Key advantages of MuPrime tumor homografts include:
Our tumor homograft models are well characterized with available characterization data including:
Visit MuBase for all available model data
MuScreen Efficacy and PD Mode are run following a preset schedule along with a shared vehicle group.
The main study endpoint for the efficacy mode is Tumor Growth Inhibition (TGI) with optional terminal Mouse I/O RNA-Seq Panel readout, FACS and IHC.
In the PD mode, choose between FACS analysis of tumor infiltrating lymphocytes and tumor associated macrophages at selected time points, and Mouse I/O RNA-Seq Panel which comprehensively profiles 1080 genes associated with tumor immunity, as your main endpoint. Additional endpoints include cytokine panel profiling in blood and tumor, IHC for biomarker analysis on tumor tissues, blood cells, lymph nodes, and spleen cells.
Frozen or fixed tumors are available on request for both modes.
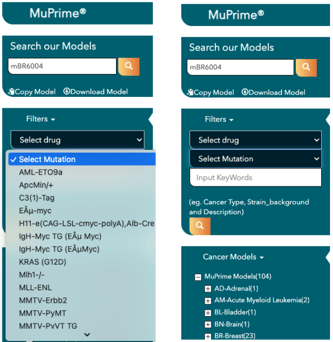
Find the best models for the preclinical evaluation of your immuno-oncology agent by logging on to Crown Bioscience’s searchable database of well-characterized and validated mouse models. Crown Bioscience offers a wide selection of murine research models including syngeneic, tumor homograft (MuPrime), GEMM, and humanized drug target (HuGEMM) models to screen your compounds of interest.
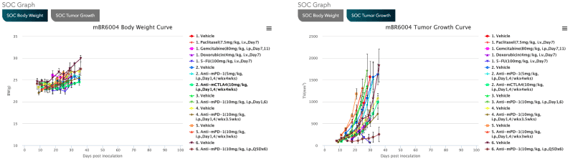
Access MuBase to explore genomic, molecular, and phenotypic data including tumor growth curves, standard of care response, histopathology data, and immune cell profiling data on this wide collection of immuno-oncology mouse models.
Browse, search, and stratify across different model types
Review treatment data for specific models including immune checkpoint inhibitors
Interested in enrolling your Immuno-Oncology Agents? or have more questions?
© 2024 Crown Bioscience. All Rights Reserved.


© 2024 Crown Bioscience. All Rights Reserved. Privacy Policy
2024-11-12
2021-10-22
site_page