3D Ex Vivo Patient Tissue Platform
Moving Immuno-Oncology Models Closer to the Clinic
Despite advances in both 3D in vitro human cell-based models and in vivo animal models, 97% of oncology drugs that enter clinical trials fail to receive regulatory approval. To overcome this poor approval rate, we need patient-relevant translational systems that better mimic the heterogeneity and molecular/genetic complexity of human tumors to:
- Understand drug effects on the native tumor microenvironment (TME)
- Gain more accurate insights beyond general cell viability (i.e. CTG)
- Evaluate immuno-oncology (I/O) drugs including immune checkpoint inhibitors (ICI) with endogenous immune cells
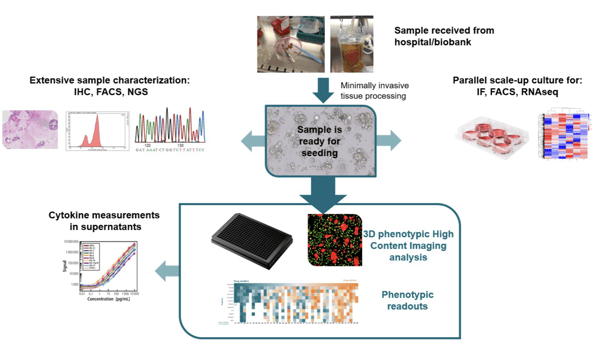
The 3D ex vivo patient tissue platform is a highly translatable screening assay for evaluating monotherapy and combination drug responses in patient tumors with endogenous immune cell populations intact. Includes the option to use in co-culture with autologous immune cells.
A Unique 3D Ex Vivo Patient Tissue Platform
Contact us to discuss your 3D Ex Vivo Patient Tissue project
Advancement Over Traditional Models
In recent years, the TME has been shown to play a crucial role in cancer progression and metastasis as well as treatment response. However, there has been a lack of research models that accurately represent the human TME. Challenges associated with traditional models include:
- In vitro assays, such as 2D cell lines, lack the complexity and heterogeneity of patient-derived tumors and its TME.
- In vivo models, such as PDX and GEMM, are more complex to develop and have lower throughput.
The 3D ex vivo patient tissue platform provides an alternative approach by keeping the complexity of the tumor (3D assay) and including the patient autologous TME into the assay.
Preserving Patient Tissue Biology
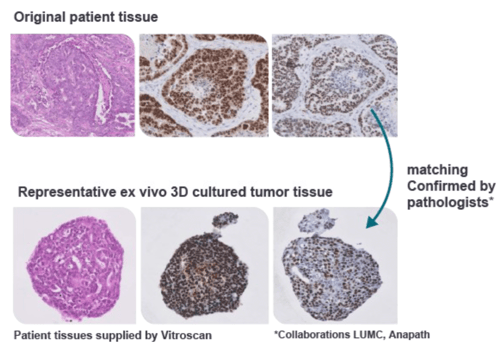
Make better informed decisions about progressing compounds/therapeutic candidates into the clinic with the most clinically relevant ex vivo patient tissue platform.
Comparison of Models
poor (x), medium (~), high (√)
| Attributes | 2D cultures | Organoids | In vivo | Ex vivo |
|---|---|---|---|---|
| Physiological complexity | X | ~ | ||
| Throughput | X | ~ | ||
| Translatability to the clinic | X | ~ | ~ |
Contact us to discuss your 3D Ex Vivo Patient Tissue project
Features
- Physiologically relevant 3D models. Understand immuno-oncology therapeutic effects on fresh patient tissue with native TME in 3D which is the most physiologically relevant preclinical environment. Preserves native TME with endogenous immune cells, fibroblasts, and other stromal components. From patients to assay plate within 24 hours.
- Multiple readouts. Select from a variety of readouts including high content imaging for efficient combination and dosing regimen evaluations, and flow cytometry for in depth evaluation of the immune population.
- Co-culture with autologous PBMCs available. Test your compound efficacy in an enhanced TME.
- Reliable, reproducible data. Tumor killing and immune cell proliferation are accurately measured via phenotypic analysis to support important R&D decisions.
- Customizable 384-well plate format. Patient-specific plate: 50-300 patient tumor tissues directly seeded in hydrogel matrix in 384-well format.
Applications
Ex vivo testing protocols established for a wide range of solid tumors representing the complexity of the TME and preserving patient tumor biology.
- Mono- and combination therapy assessment, including ICI evaluation in vitro:
NSCLC Patient Samples Response to ICI Correlates With Clinical Data
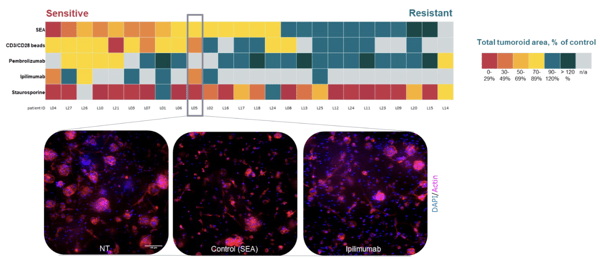
Heatmap showcasing the differential response of 25 NSCLC patient samples to a set of SoC treatments including pembrolizumab and ipilimumab.
- Late-stage preclinical candidate evaluation
Cytokine Analysis Confirms HCI Results
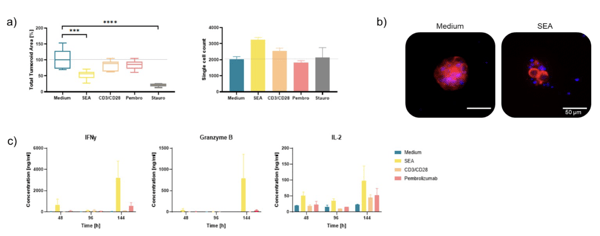
a) Ex vivo tumor tissue from NSCLC patient responded strongly to SEA and moderately to CD3/CD28 beads and pembrolizumab treatment (6 days).
b) NSCLC tumoroid in medium only and SEA conditions (DAPI: nuclei; TRITC: actin) c) HCI response measurements were confirmed by cytokine analysis
at various timepoints (2, 4, and 6 days). The increase in IFNgamma, Granzyme B, and IL-2 upon IO treatments is supporting the
immune-mediated killing mechanism
- Potential for identifying responder/non-responder profiles
Differential Responses to SoC Drugs in Breast Cancer Patient Tissue Samples
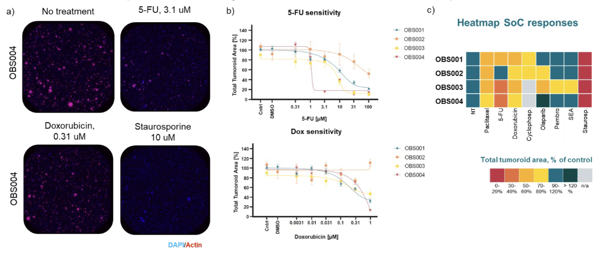
a) Example images of OBS004 response to different treatment conditions. b) Patient-specific sensitivity (4 breast cancer samples) to a
6-point concentration curve of 5-FU and doxorubicin c) Heatmap illustrating SoC response to a set of standard of care drugs.
- Immune response testing in patient tumors with enhance TME
- Potential for selection of indication for clinical trials
Readouts
Multiple readouts available including:
- High Content Imaging: functional assay assessing phenotypic effects of the (immune)-oncology compounds on ex vivo patient cultures in 3D
- FACS: to gain insights on immune niche composition of the primary samples. Up to 10 marker panels can be tailor-made specifically to your project needs.
- IHC: to confirm tumor, stromal and immune content in the initial samples as well as to analyze specific target expression
- Cytokine analysis: to gain an in depth cytokine, which can be indicative of cellular processes before these take effect in functional readout.
- Sequencing: to address genetic and transcriptional profiles of the primary ex vivo samples.
Our ex vivo patient tissue platform is one of several high-content imaging-based screening assays offered. These include:
- Immuno-oncology assays
- Oncology assays (cell lines and organoids)
Contact us to discuss your 3D Ex Vivo Patient Tissue project.