- Our Services
- Platforms
- Target Solutions
- Technologies
- Service Types
- Our Science
- About Us
- Contact us

Rapidly evaluate the pharmaceutical and safety properties of your new agents with our in vivo pharmacokinetic (PK) services. Use our pharmacological and bioanalytical DMPK platform to select the most robust drug formulations, and optimize drug discovery and preclinical development strategies.
Our non-GLP DMPK services are available as an integrated drug discovery platform or a standalone service, and include cutting-edge LC-MS/MS and HPLC technologies alongside ELISA-based and MSD assays.
| In vivo PK of small and large molecules |
|---|
|
| Bioanalysis |
|---|
|
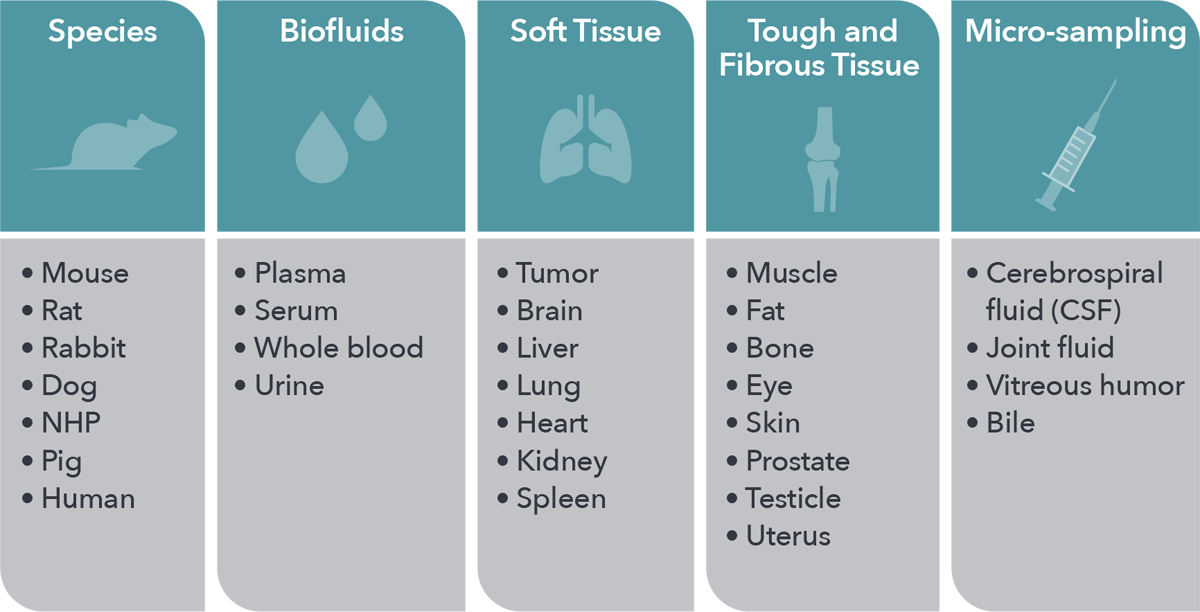
Inform your drug development by leveraging our unparalleled in vivo expertise and models, with a range of animal species and tissue types amenable to all in vivo PK needs. High quality data is assured for every study through extensive quality assurance systems.
Tell us about your needs
© 2024 Crown Bioscience. All Rights Reserved.


© 2024 Crown Bioscience. All Rights Reserved. Privacy Policy
2024-11-05
2021-10-22
site_page