 Prostate cancer is a particularly difficult cancer type to model for preclinical research. Why are prostate cancer models so challenging, and how can researchers deliver clinically-relevant results?
Prostate cancer is a particularly difficult cancer type to model for preclinical research. Why are prostate cancer models so challenging, and how can researchers deliver clinically-relevant results?
Complex Pathogenesis of Prostate Cancer
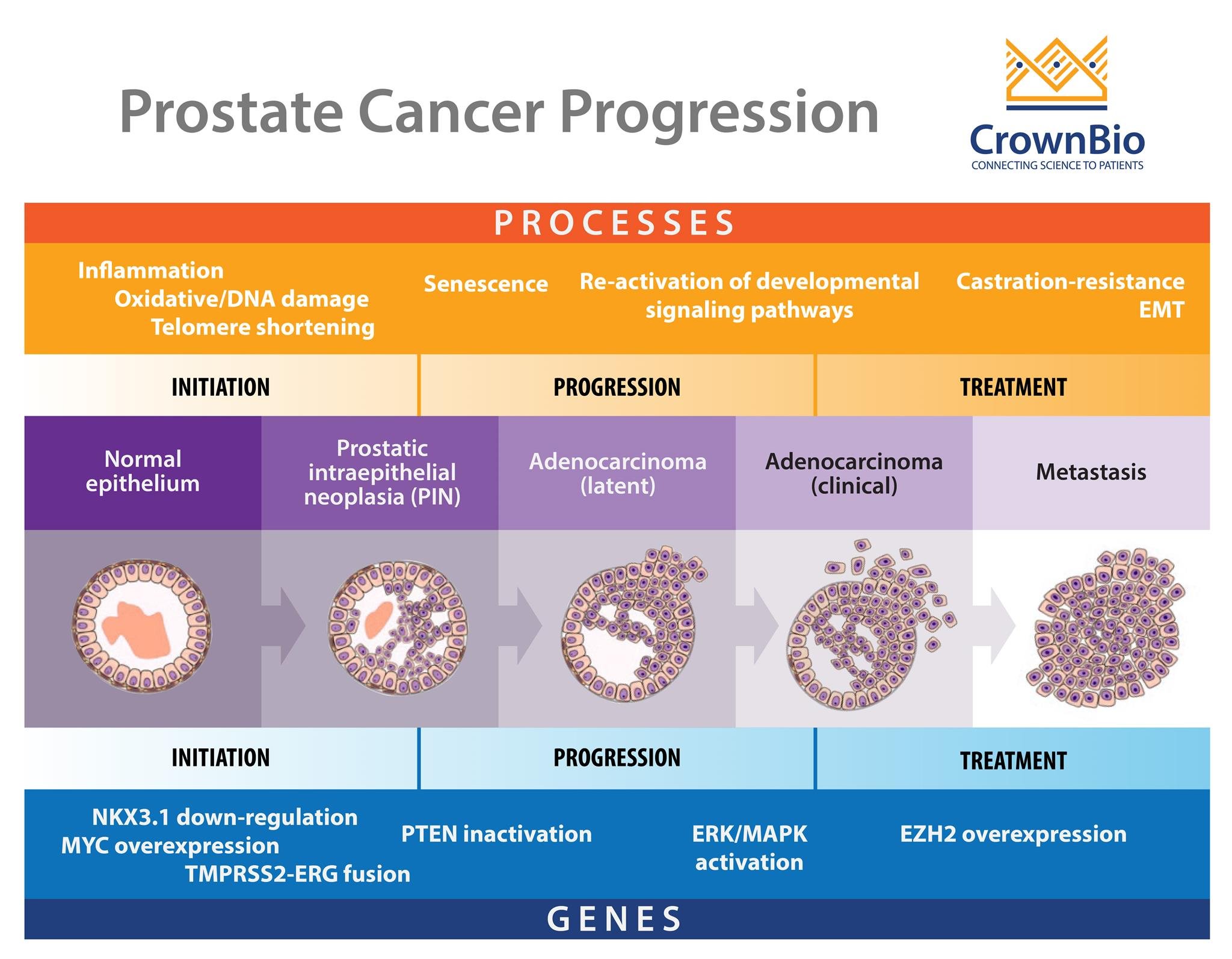
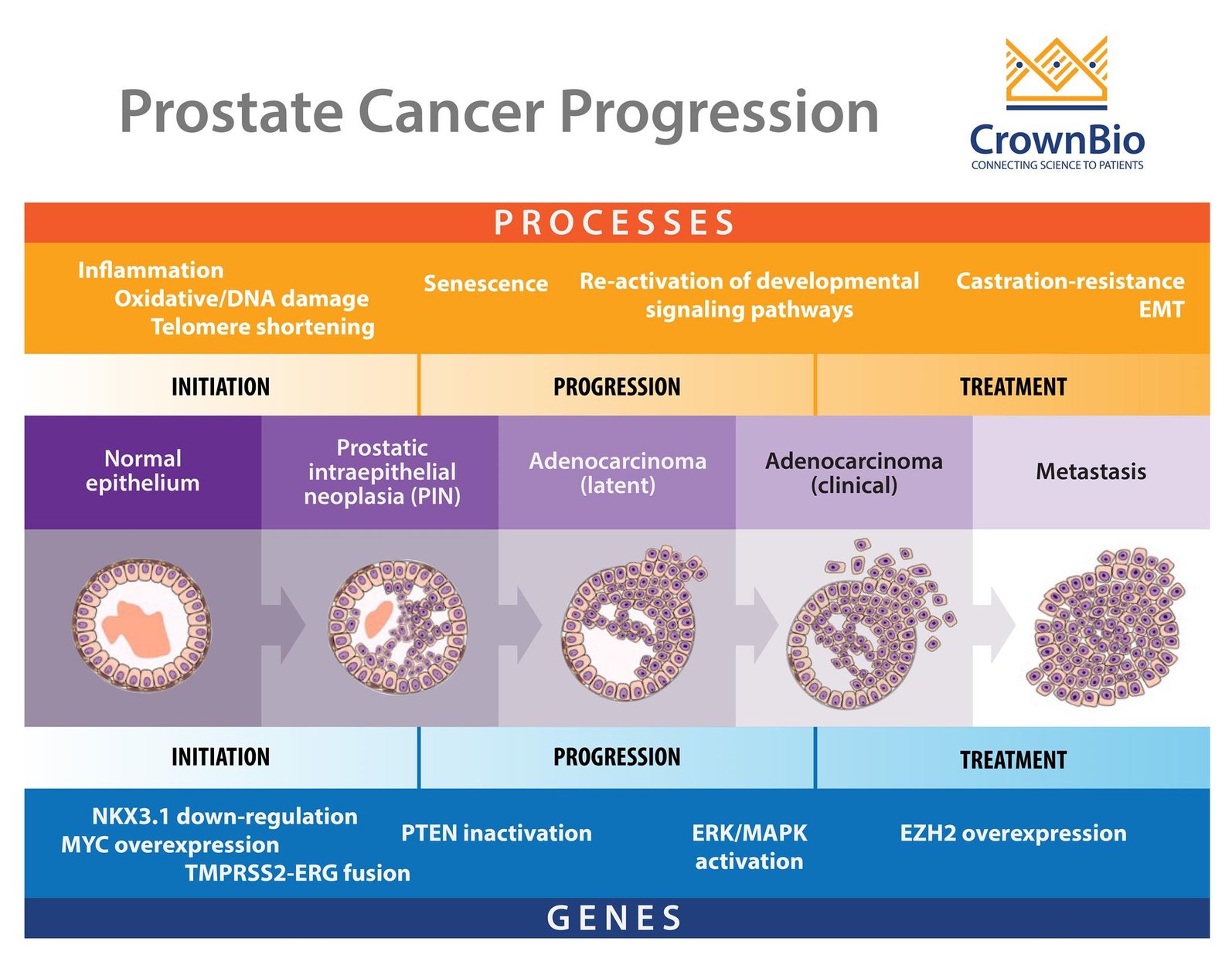
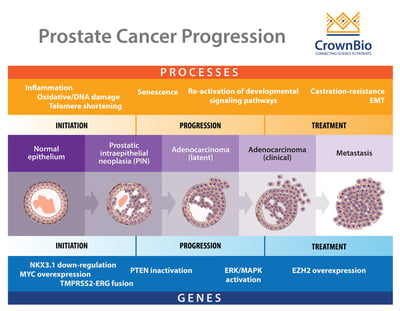
One of the main difficulties in prostate cancer modelling is the complex pathogenesis of the disease itself. It is a progressive, multistage disease, with many stages having different molecular features, drug response, and resistance mechanisms.
Modelling Castrate Sensitive and Resistant Prostate Cancer
To start with, there’s the two main stages of disease – castrate sensitive and castrate resistant -- which themselves can be metastatic or nonmetastatic. This means that evaluating one agent for both disease stages may require multiple models. This becomes more complex within the disease stages, as with castrate resistant prostate cancer (CRPC).
There’s another wide spectrum of disease within this subtype - some patients may have rising PSA levels and disease progression, others may have a dependency on androgens, while metastasis can also occur. So, again, specific models are needed group by group if you want to develop a hormonal-targeted agent and test it on models which do and don’t have a dependency on androgens.
Evaluating Multiple Molecular Targets
Within subtypes, there are varying, clinically-relevant molecular features which may need to be targeted. During initiation of prostate cancer, genetic events such as PTEN inactivation and somatic gene fusions between TMPRSS2-ERG take place. As the disease progresses, ERK/MAPK pathway mutations are seen, with EZH2 overexpression occurring during metastasis.
All of these features also need to be modelled effectively to target the disease and understand how they affect treatment response.
Wide Variety of Resistance Mechanisms
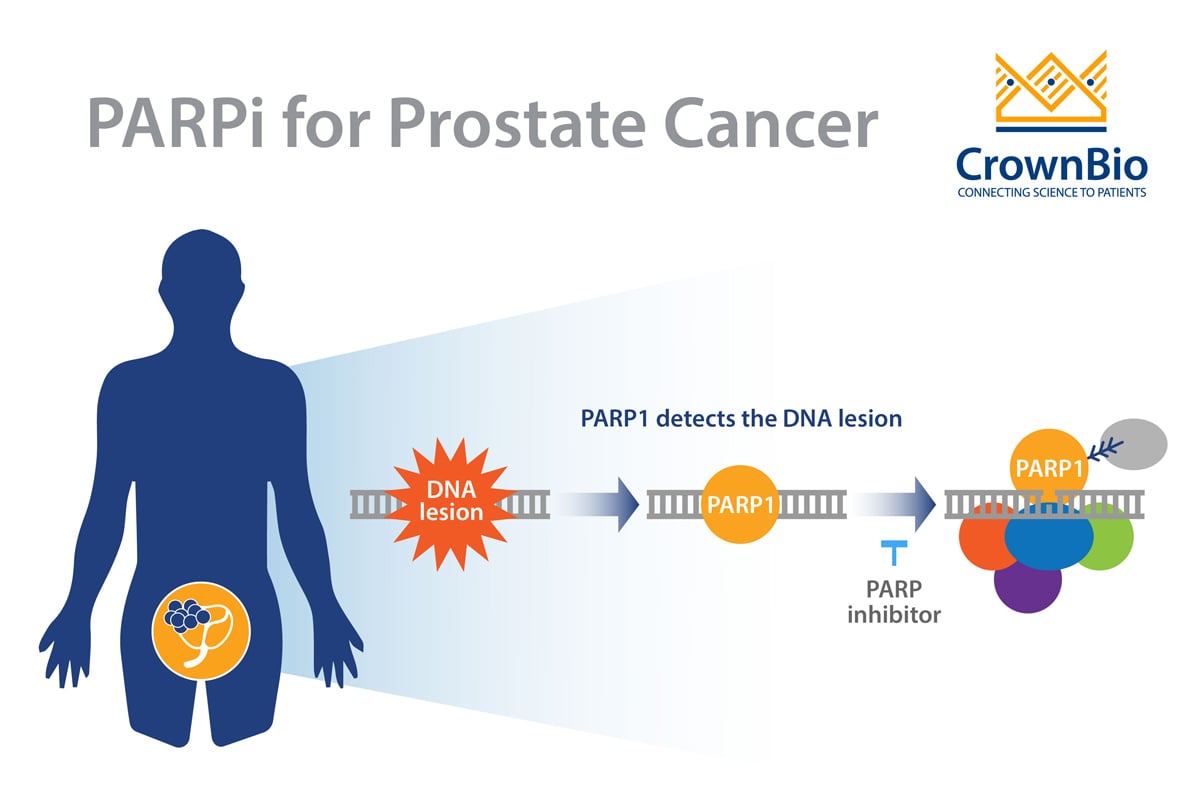
Then there’s late-stage resistance mechanisms. Newer, hormonal-targeted agents such as enzalutamide and abiraterone have provided new treatment options for metastatic CRPC. But around 25% of patients have de novo resistance to these agents, while others can develop resistance.
Unfortunately, this is due to more than one mechanism. Some resistance mechanisms to these agents are unique, while others are linked to how the disease has progressed. They can be split by androgen receptor-dependent and independent mechanisms, which again means different treatment options would be needed per group -- and different preclinical models.
It also means that combination therapies are likely to be a way forward in treatment, as is proving true for many other cancers. This adds further complexity to preclinical prostate cancer research, as models are needed which are suitable to test new and existing agents in combination regimens.
Current Preclinical Prostate Cancer Models
Just thinking about oncology studies (and excluding immuno-oncology, which requires immunocompetent models), there are effective xenograft tumor models available for prostate cancer research. But while our understanding of prostate cancer has increased, in terms of the proteins/pathways to target, resistance mechanisms, and disease heterogeneity, the diversity of available prostate cancer models hasn’t.
Most historical prostate cancer studies have been carried out with just three prostate cancer cell lines: PC-3, DU-145, and LNCaP. LNCaP alone has been referenced in more than 7,000 publications in PubMed.
However, all three lines/models have limitations; DU 145 and PC-3 don’t express PSA, and LNCaP has a mutated, though still functional, androgen receptor. While these and other prostate cancer cell lines have good pharmacological characterization for in vitro and proof-of-concept studies, they provide poor biological characterization for in vivo studies.
Shortfalls of Cell-Line Derived Xenografts
None of these approaches accurately model clinical disease progression from local to advanced disease, resistance mechanisms to a range of treatments, or appropriately mimic patient disease. As well as prostate cancer being a complex disease, part of the problem is due to the inherent nature of “traditional” cell line derived xenografts. As they adapt to many years growing on plastic, xenografts drift from original disease, making them less relevant model options.
What About Patient-Derived Xenografts?
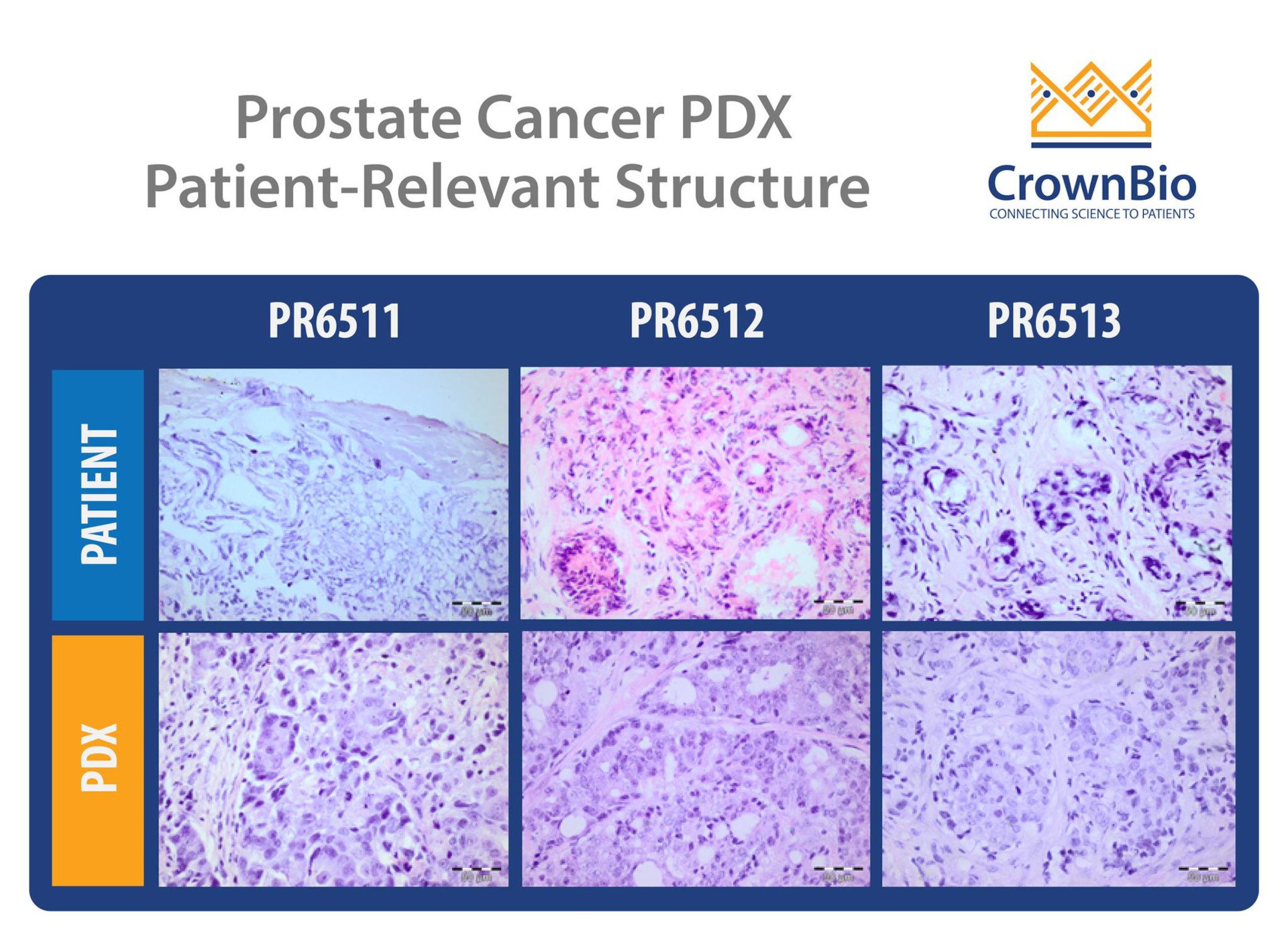
Patient-derived xenografts (PDX), derived directly from patient tumors and never adapted to grow in vitro, are known to be a more predictive preclinical option. As they capture more of the patient heterogeneity and better reflect clinical phenotypes and genotypes, they should provide an alternative to cell line derived xenografts.
Developing PDX from prostate cancer is trickier than for other indications, however, due to issues with generation and propagation. Development of prostate cancer PDX are known to be time consuming and labor intensive, with low take rates.
Model panels are being generated, but these tend to be at much lower numbers then for other cancer indications.
What Next for Preclinical Prostate Cancer Research?
Patient-derived models, and newer technologies such as organoids, continue to offer more patient relevant tools by capturing more of prostate cancer’s complexity. As our understanding of prostate cancer continues to grow, we need further development of these models to provide more resources which better recapitulate the complex human disease.
This will enable the development of more agents targeting the multiple stages and genetic features which drive prostate cancer, and hopefully more clinically approved treatment options for patients.