Drug Development With Organoids
Advance oncology research with HUB Organoids™.
Our proprietary models deliver reproducibility, standardized assays, and unmatched turnaround time to move your program forward faster.
Make Faster, Smarter Research Decisions
These organoid platform features help you reduce the risk of clinical failure by
generating data that is more predictive, reproducible, and clinically relevant.
-
Turn organoid complexity into speed with assay-ready organoid batches that deliver CTG efficacy data across 50 organoid models in only 3 weeks.
-
Trust that results reflect patient biology because our proprietary culture methods, built on exclusive Clevers Lab HUB protocols, preserve clinical fidelity that standard systems lose.
-
Expand into new therapeutic areas with confidence by accessing organoids from more than 30 organ and tissue types, enabling cross-indication studies and comparative analysis.
-
Make program decisions with reliable, reproducible data thanks to standardized assays that minimize variability and streamline large-scale screening.
-
Uncover the mechanisms behind response and resistance using RNAi and CRISPR/Cas tools to validate targets, explore pathways, and discover biomarkers.
-
Gain deeper insights than endpoints alone can offer through advanced 3D imaging that reveals how cells interact and change dynamically under treatment.
-
Move from discovery to validation more quickly with matched in vitro PDXO and in vivo PDX models that ensure continuity and reduce translational gaps.
-
Scale studies without losing clinical relevance because PDOs and PDXOs preserve patient complexity and expand indefinitely for both focused and high-throughput projects.
-
Select the right models faster and more effectively using our living biobank, enriched with microscopy, genomic, transcriptomic, and pharmacology data.
-
Strengthen multimodal development strategies earlier by testing drug radiation combinations in organoids before committing to in vivo or clinical studies.
Platform Applications
Test candidate efficacy, safety, side effects, and pharmacokinetic properties as well as mechanism of action.

Evaluate safety and toxicity profiles using healthy organoids developed from non transformed cells.

Capture disease relevance and patient population heterogeneity with disease modeling, including models of drug resistance.
Identify your target patient population before entering the clinical trials.

Determine potential biomarkers of therapeutic response with companion diagnostics.
Study Options
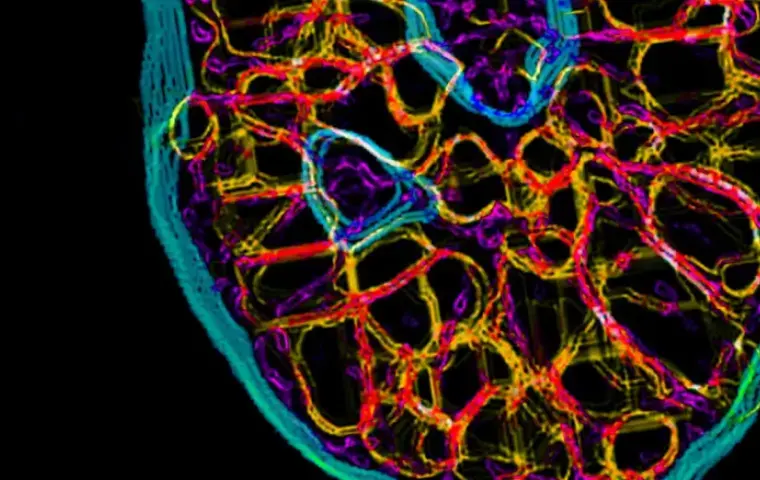
Select models and evaluate efficacy and multi-drug combination strategies.
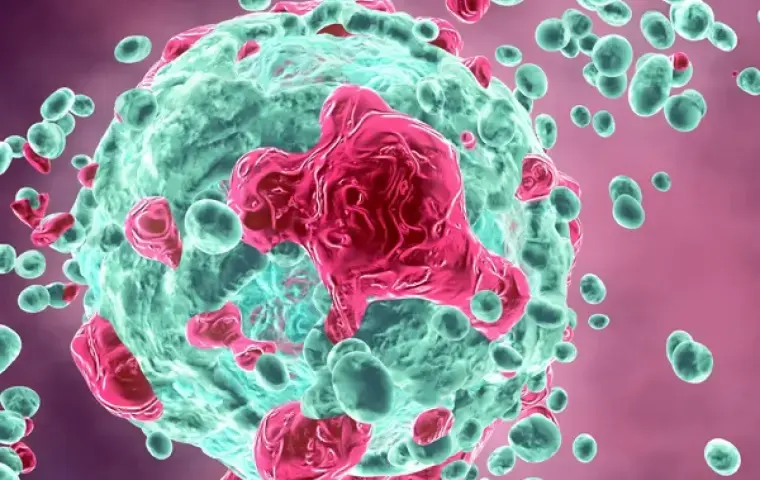
Identify responder and non-responder tumor models. Leverage high content services to produce highly comprehensive morphology readouts in both 2D and 3D models. Use multifactorial data for downstream lead ID and decision-making.
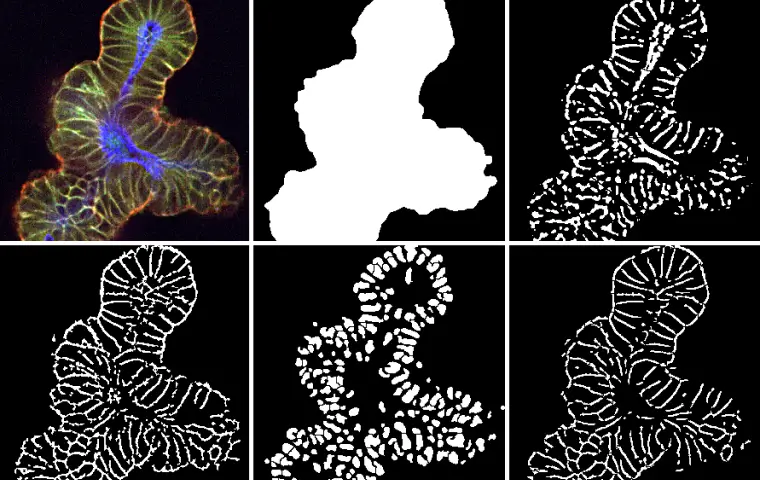
Phenotypically screen up to thousands of compounds. Layer and score drug effects with image mediated screens.
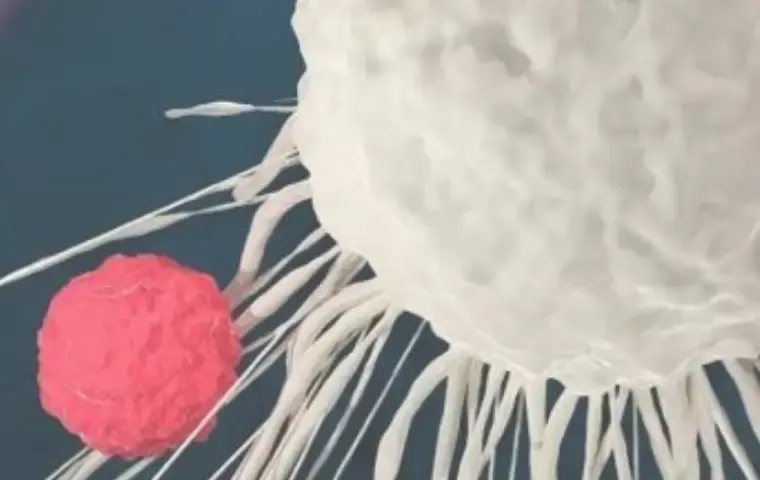
Benefit from the only immuno-oncology platform incorporating patient-relevant 3D in vitro models co-cultured with non-autologous immune cells.
Predictive, Scalable 3D Organoid Models for Diverse Patient Representation
Make use of the great translatability of our 3D organoid models, which show unprecedented patient response predictivity. Benefit from faster development and enhanced scalability of organoids. Model and mirror patient population diversity via screening multiple tumor organoid models simultaneously, and access both tumor and healthy organoids to evaluate in vitro data with clinically relevant drug potency and efficacy as well as off-target effects.
Frequently Asked Questions
HUB Organoids™ are created using Clevers Lab protocols through exclusive partnerships to ensure high fidelity, reproducibility, and proprietary culture methods for oncology drug development.
We offer paired in vitro PDXO and in vivo PDX models, allowing for a seamless transition between study phases and improved translational predictivity.
OrganoidBase™ is our searchable organoid database containing genomic, transcriptomic, and pharmacology data to streamline model selection.
Yes. Our biobank includes both tumor organoids and healthy organoids from non-transformed cells, enabling safety and toxicity profiling alongside efficacy testing.
Organoids derived from patient tumors often provide higher translational predictivity for certain therapeutic classes because they retain the genetic and phenotypic characteristics of the original tumor.
Yes. We can establish new patient-derived organoids from your own samples or collect fresh tumor material through our network of partners, subject to ethical approvals.
Discuss Your Target ID and Validation Project
From in vitro and in vivo models to in silico insights, genomics, proteomics, and exclusive biobank resources, we deliver the integrated solutions you need to discover, validate, and advance the next generation of therapies. Contact us today to accelerate your breakthroughs.