Humanized and Genetically Modified Mouse Models
Predict clinical success by testing IO therapies in models that restore human immune function and replicate patient-like interactions, delivering answers standard mouse systems can’t.
Why Humanized Mouse Models?
They allow direct in vivo evaluation of human-specific immunotherapies, which traditional murine systems can’t achieve. By engineering mice to express human drug targets like checkpoint proteins within a fully functional immune system, these models deliver a translationally relevant and efficient alternative to fully humanized systems. They are particularly powerful for studying antibody combinations, immune memory, and checkpoint inhibitors.
Humanized mouse models enable teams to:
- Study a broad range of human immunotherapy targets in vivo using humanized receptors and ligands.
- Evaluate agents when murine orthologs are non-functional or absent, ensuring target engagement.
- Reduce cost and complexity compared to human immune system (HIS) models while preserving mechanistic insight.
Generation of Humanized Target Tumor Bearing Models for Immunotherapy Evaluation
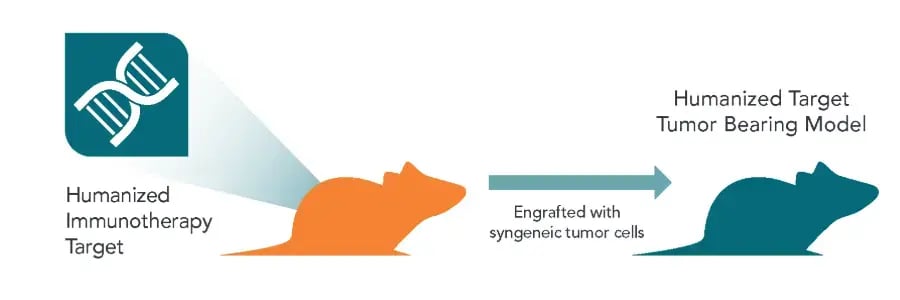
Model Highlights
Evaluate single or dual targeted immunotherapies using validated humanized immune models and tumor lines engineered to express human ligands.
Single and double knock-in models expressing human PD-1, PD-L1, CTLA-4, and others. Support in vivo testing of FDA-approved and novel immune checkpoint inhibitors.

Syngeneic tumor cell lines are engineered with human ligand expression (e.g., MC38-hPD-L1) for modeling dual interactions between tumor and immune components.
Humanized Mouse Models for IO Drug Development
Watch this webinar to learn about:
- Humanized mouse models for in vivo oncology studies
- Evaluating IO therapies in humanized mice
- Engraftment of cell line (CDX) and patient-derived xenografts (PDX) in humanized mouse platform
Frequently Asked Questions
Syngeneic models are syngeneic tumor lines expressing human ligands, ideal for evaluating dual-target interactions when used with humanized, genetically modified mouse models.
Crown’s portfolio includes PD-1, PD-L1, CTLA-4, CD137, TIGIT, OX40, LAG3, and others, with continuous pipeline expansion.
Applications include in vivo validation of checkpoint inhibitors, combination strategies, immune memory studies, and immune infiltration analysis via TIL and FACS.
Compared to fully humanized immune system mice, these models are more cost-efficient, faster to establish, and require fewer specialized resources while maintaining high translational relevance.
Standard efficacy endpoints can be combined with immune profiling (flow cytometry, TIL analysis), biomarker discovery, and pharmacodynamic readouts to provide a comprehensive evaluation.
Ready To Start
Your Study?
Start Translating IO Faster