Reliable CDX Models for Oncology
Evaluate drug efficacy, PK/PD, and resistance with an expansive CDX library and specialty formats for fast, translational oncology research.
Accelerate Drug Development
Access over 200 validated CDX models across solid and hematologic malignancies, with options for orthotopic, systemic, and dual-tumor designs. These models support rapid transitions from in vitro screens to in vivo efficacy, PK/PD profiling, biomarker analysis, and resistance studies. Advanced imaging, customizable implantation formats, and humanized compatible models enable tailored study designs for preclinical decision-making.
With our platform, you get:
-
Large-scale model library with short turnaround and robust historical data
-
High reproducibility for comparative or combination drug efficacy studies
-
Seamless transition from in vitro screening to in vivo validation
Explore multiple in vivo assay systems to evaluate novel anticancer compounds, with each assay specifically designed to understand a drug's mechanism of action or an individual agent property.
Cell Line-Derived Xenograft Features
Our CDX models are optimized for scalability, reproducibility, and translational relevance across oncology programs.

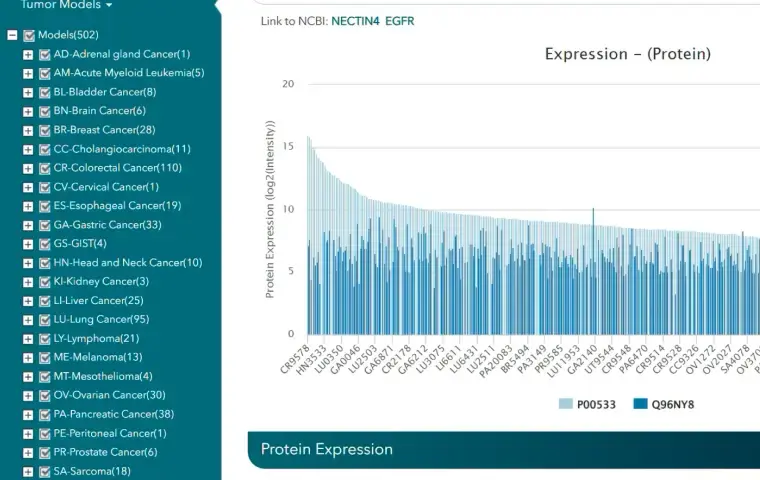
Over 200 models are available across solid and hematologic cancers, with engineered variants and orthotopic, systemic, and dual-tumor formats. The searchable CDX model database allows filtering by tumor type, mutation status, or response profile.
CDX studies can be initiated based on results from in vitro cell line screens using the same cancer cell lines, enabling a seamless transition from high-throughput discovery to in vivo validation.
These models include PDX-derived cell lines and dual xenograft systems for simultaneous efficacy and toxicity evaluation.
Study Options
Our design studies range from lead ID to resistance modeling using validated CDX tools and endpoints.
Evaluate single agent and combination drug efficacy in solid and hematologic tumors.

Develop custom drug-resistant CDX models via CRISPR or chronic dosing to evaluate second-line therapies and uncover resistance mechanisms.
In vivo imaging-enabled (BLI and Ultrasound) orthotopic and systemic model studies are available to simulate advanced disease settings and to noninvasively monitor tumor burden, metastasis, and treatment response in real time.

Evaluate anti-tumor activity using PDX, CDX, and organoid models in combination with alpha- and beta-emitting isotopes. Supported by MDC’s radiochemistry expertise, biodistribution analysis, and comparator studies with approved therapies.
Beyond Checkpoints: Strategic Use of CDX Models in Emerging IO Therapies
Watch our webinar to learn how to:
- Enhance tumor microenvironment (TME) in CDX models to better replicate immune-tumor interactions.
- Create humanized mouse models by engrafting human immune cells to allow for IO therapy evaluation in a human immune context.
- Use CDX models to refine dosing schedules for immunotherapies and combination treatments.
- Utilize multiple patient-derived cell lines and genetic modifications to mimic heterogeneity and study therapy responses across different genetic backgrounds.
- Perform serial biopsies and liquid biopsies in CDX models to identify mutations and biomarkers linked to resistance.
Frequently Asked Questions
Understand what makes CDX models a scalable, go-to platform for translational oncology studies.
CDX models are used to evaluate drug efficacy, pharmacokinetics, resistance, and biomarker expression in vivo, supporting both early-stage screening and IND-enabling studies.
Subcutaneous, orthotopic, systemic, and dual xenograft options are available for modeling primary and metastatic disease.
Yes, selected CDX models are compatible with PBMC or CD34+ humanized mouse platforms for evaluating IO agents.
Crown Bio’s searchable CDX and cell line databases allow filtering by tumor type, mutation, or drug response profile.
Available endpoints include tumor growth curves, survival analysis, histopathology, biomarker analysis, immune infiltration (for humanized studies), biodistribution, and in vivo imaging.
Ready To Start?
Connect with us to select the right CDX model and study format for your preclinical goals. Design your CDX study today.