3D Bone Marrow Models: Transforming Drug Discovery for Hematopoietic Disorders
Revolutionizing Drug Development with Advanced 3D Bone Marrow Models
Bone marrow is the primary site of hematopoiesis, where hematopoietic stem and progenitor cells (HSPCs) differentiate into blood and immune cells. This tightly regulated process is influenced by stromal support cells, extracellular matrix components, and vascular endothelial cells. Disruptions to this microenvironment can lead to hematological disorders, immune deficiencies, and malignancies, creating a critical need for more predictive preclinical drug testing platforms.
While traditional scaffold-free hematopoietic stem cell cultures have been widely used, they fail to replicate the complex three-dimensional (3D) bone marrow niche, failing to support primary cell proliferation - leading to limited translational success for drug candidates. Next-generation 3D bone marrow models provide a more physiologically relevant system for studying hematopoiesis, screening drug candidates, and evaluating novel therapies for leukemia, myeloma, and bone marrow malignancies.
Bridging the Gap Between In Vitro Models and Clinical Outcomes
One of the key challenges in hematopoietic drug development is the lack of reliable in vitro models that accurately predict drug efficacy and toxicity. Conventional growth factor-based cultures fail to sustain long-term HSPC function, and while stromal feeder layers provide some support, they lack the structural complexity of the native bone marrow microenvironment which is critical to recreating cancer cell drug resistance.
This deficiency in physiologically relevant in vitro models hinders early-stage hematopoietic drug discovery, making it challenging to assess cell-cell and cell-matrix interactions, therapeutic responses, and toxicity accurately. As a result, researchers face difficulties when transitioning from cell lines to patient-derived samples or in vivo models, increasing the risk of late-stage drug failure and limiting the development of effective hematopoietic therapies.
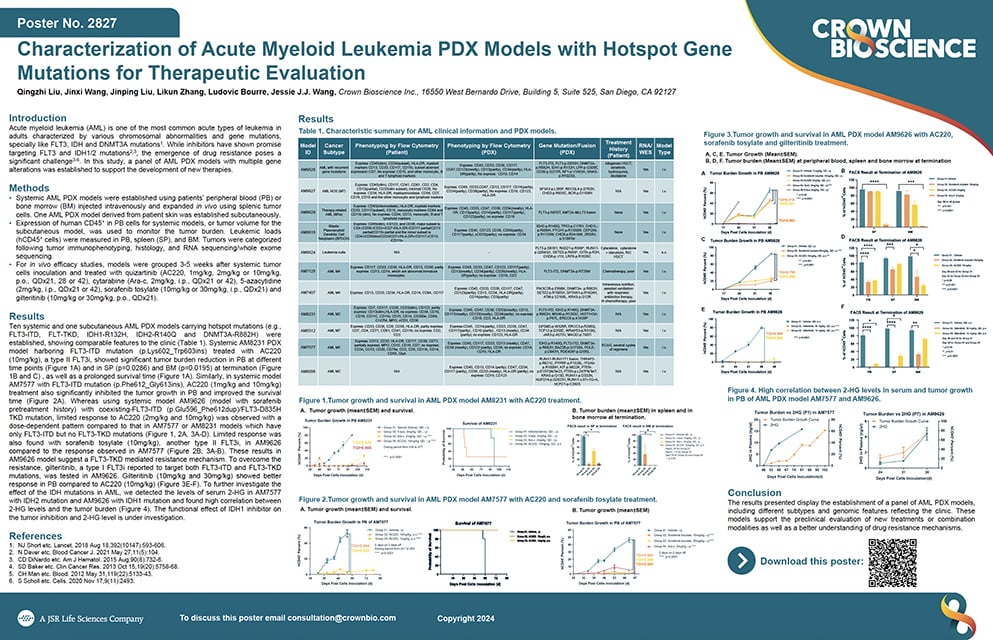
Advancing Targeted Therapies: 3D Bone Marrow Niche and PDX Models for AML and Multiple Myeloma Research

Talita Stessuk
Scientist II, BMN Platform Lead

Gerold Feuer, PhD
Senior Director, Scientific
Research & Innovation
To address these limitations, Crown Bioscience has developed an advanced 3D in vitro bone marrow model using mesenchymal-endothelial networks that are an optimized in bio-active hydrogel. This system:
- Supports HSPC expansion
- More accurately models drug responses compared to traditional suspension cultures
- Recreates the stromal-rich bone marrow niche, including mesenchymal and endothelial compartments
- Is compatible with diverse tissue sources including cell lines and patient samples
- Integrates with autologous and heterologous immune cells
- Reduces reliance on animal models in preclinical testing
- Improves translation from in vitro to in vivo studies
Our 3D bone marrow models offer highly predictive platforms for:
- Hematopoietic toxicity testing: Evaluating drug-induced effects on stem cell survival.
- Leukemia and myeloma drug screening: Testing targeted therapies in a disease-relevant bone marrow microenvironment.
- Cell and gene therapy development: Assessing the efficacy of stem cell-based treatments and genetic modifications.
- Immuno-oncology research: Studying interactions between immune cells and hematological malignancies in a realistic setting.
The Future of Hematological Cancer Drug Development
As 3D cell culture technologies continue to evolve, they are redefining preclinical drug testing by providing more accurate, reproducible, and scalable models of human tissues. By integrating 3D bone marrow models into the drug development pipeline, researchers can accelerate the discovery of new treatments for blood cancers, immune disorders, and bone marrow failure syndromes, ultimately improving clinical outcomes.
Why Choose Crown Bioscience for Your Bone Marrow Niche Models?
- Cutting-edge 3D bone marrow models: Our advanced in vitro models closely replicate the native bone marrow microenvironment, providing more predictive and clinically relevant data for drug discovery and hematopoietic research.
- Accelerated drug development: By improving preclinical screening accuracy and smoothly transition studies from in vitro to in vivo our models help de-risk drug candidates and speed up the path to clinical trials.
- Customized solutions for targeted research: We offer tailored 3D bone marrow models designed to meet specific research needs, including disease-specific models, patient-derived cultures, and IO applications.
- Expertise in hematopoietic and oncology research: Our team brings deep scientific expertise in stem cell biology, hematological malignancies, and drug screening, ensuring high-quality data and actionable insights for biotech and pharmaceutical partners.

Seamless Translation from 3D Bone Marrow Niche Models to In Vivo Models
Our 3D bone marrow niche models bridge the gap between in vitro drug screening and in vivo validation, providing a physiologically relevant microenvironment that enhances translational success. These models seamlessly integrate with our robust portfolio of in vivo models, allowing researchers to assess drug efficacy, resistance mechanisms, and therapeutic responses with greater accuracy.
- Biologically relevant 3D microenvironment enhances therapeutic response evaluation and mimics bone marrow-driven resistance mechanisms.
- Models are compatible with cell lines and patient-derived tissue, improving data translation between studies.
- High-content imaging analysis provides insights into tumor killing, immune cell interactions, and potential off-target effects on niche components.

Partner with Us for End-to-End Support
Crown Bioscience offers advanced bone marrow niche models and high content imaging analysis to accelerate hematology drug development. Our 3D models provide key insights into stem cell behavior, disease progression, and drug efficacy. Speed up hematology therapy development with our end-to-end preclinical support. Reach out today to explore how we can support your bone marrow research and help advance your drug discovery efforts.
Discuss your bone marrow niche project
For Researchers Interested in Acute Myeloid Leukemia and Multiple Myeloma Models
By combining our 3D bone marrow niche models with our comprehensive Acute Myeloid Leukemia and Multiple Myeloma in vivo portfolio, we provide a powerful preclinical-to-clinical platform, ensuring faster, more reliable drug development outcomes.
FAQ: 3D Bone Marrow Niche Models
3D bone marrow models are advanced in vitro systems that replicate the bone marrow microenvironment, allowing researchers to study hematopoietic stem and progenitor cells (HSPCs) in a physiologically relevant setting. These models are essential for drug discovery because they provide more accurate predictions of drug efficacy, toxicity, and mechanisms of action, particularly for blood cancers, hematological disorders, and immunotherapies.
3D bone marrow models improve drug screening by simulating the hypoxic, vascularized niche of the bone marrow, which is essential for accurate drug response predictions. Unlike 2D cultures, which lack structural complexity, 3D models provide a more realistic environment for cell-cell interactions, stem cell differentiation, and hematopoietic function, leading to improved screening accuracy for hematopoietic drugs and chemotherapies.
While 3D bone marrow models significantly reduce reliance on animal studies, they do not entirely replace them. These models serve as a human-relevant in vitro platform for evaluating hematopoietic toxicity and drug efficacy, offering better predictive value for early-stage drug testing. However, some aspects of drug development, such as systemic effects and pharmacokinetics, still require in vivo validation.
3D bone marrow models are used to test a variety of drugs, including chemotherapies for leukemia and lymphoma, immuno-oncology therapies for blood cancers, gene and cell therapies for hematological disorders, and stem cell-based treatments. These models provide a more accurate representation of how drugs interact with the hematopoietic system, making them invaluable for preclinical drug development.
A 3D bone marrow model consists of several key components that mimic the natural hematopoietic niche. It includes bioactive hydrogels, which provide structural support similar to the extracellular matrix (ECM), as well as primary stromal cells, such as osteoblasts, adipocytes, and endothelial cells, that interact with hematopoietic stem and progenitor cells (HSPCs). Additionally, the hypoxic microenvironment is designed to enhance stem cell maintenance and differentiation, making the model a powerful tool for drug discovery and hematopoietic research.
3D bone marrow models differ from 2D cultures in several ways. They provide more realistic cell-cell interactions, better simulate the extracellular matrix and hypoxic conditions, and offer long-term support for hematopoietic stem cell maintenance and differentiation. In contrast, 2D cultures lack the structural complexity and physiological relevance of the bone marrow microenvironment, leading to less reliable drug response predictions.
Yes, 3D bone marrow models can be customized to fit specific research needs. Scientists can develop disease-specific models, such as those for leukemia or bone marrow failure syndromes, to study drug responses in a relevant setting. These models can also be tailored using patient-derived hematopoietic stem cells, allowing for personalized drug screening. Additionally, researchers can modify the extracellular matrix composition to explore how biophysical cues affect stem cell differentiation and drug efficacy.
3D bone marrow models are highly valuable in immuno-oncology research because they enable scientists to study the interactions between immune cells and hematological malignancies in a physiologically relevant environment. These models are widely used for testing CAR-T cell therapies, immune checkpoint inhibitors, and other immunotherapies for blood cancers. By closely replicating the bone marrow niche, they provide a more accurate assessment of how immune cells respond to targeted therapies.
Bioactive hydrogels play a crucial role in 3D bone marrow models by mimicking the extracellular matrix (ECM) and supporting cell adhesion, signaling, and differentiation. They help create a self-assembling stromal network that enhances the function of hematopoietic stem and progenitor cells. Additionally, bioactive hydrogels reduce the need for excessive growth factors, making drug testing conditions more cost-effective and biologically relevant.
Integrating 3D bone marrow models into your drug discovery pipeline can enhance preclinical research by improving early-stage drug screening, toxicity testing, and personalized medicine approaches. These models allow researchers to test hematopoietic drugs and chemotherapies in a system that closely resembles the human bone marrow niche, leading to better predictions of clinical outcomes. By providing more accurate data on drug efficacy and safety, 3D bone marrow models help accelerate the development of new therapies for blood cancers and immune disorders.