Find Human Biospecimen Solutions for Next-Generation Research
Crown Bioscience’s comprehensive human biospecimen solutions include FFPE and fresh frozen tissues, custom collections, and CAP-accredited pathology services to support biomarker discovery and translational research. Request a quote today.
A key challenge in oncology research is securing high-quality, ethically-sourced human biospecimens that are comprehensively characterized and preserve molecular integrity. Inconsistent sample quality and a lack of associated clinical data can lead to unreliable research outcomes and failed experiments.
That’s why Crown Bioscience provides:
- Globally-Sourced Biobank
Access to a vast, IRB-approved global network for diverse and ethically-sourced samples from various cancer indications.
- Superior Sample Integrity
Strict, standardized workflows with ultra-short ischemia times to ensure the highest quality of DNA, RNA, and protein for advanced applications.
- Comprehensive Data
Each biospecimen is accompanied by extensive, digitally-linked clinical data, including treatment history and demographics.
- End-to-End Compliance
Rigorous adherence to global regulatory standards like HIPAA, GDPR, and GCP to ensure ethical and compliant research practices.
Request a Quote
Human Biospecimen Biobank
Crown Bioscience offers a globally-sourced, IRB-approved human biospecimen biobank, purpose-built for translational and precision oncology research. Our biobank includes high-quality tumor tissue, matched adjacent normal, plasma, serum, and PBMCs—all collected under standardized, compliant protocols with ultra-short ischemia times to preserve molecular fidelity. Leveraging an international clinical network, we deliver biospecimens paired with extensive clinical data to support multiomics applications, biomarker discovery, and diagnostic assay development.
Biospecimen Quality, Compliance, and
Clinical Relevance
Crown Bioscience delivers rigorously controlled, high-quality human biospecimens collected under IRB-approved protocols and standardized workflows to ensure consistency and reproducibility. These standards safeguard biological relevance, enabling researchers to confidently advance multiomics profiling and clinical assay development.
Our experienced team ensures:
- Minimal ischemia time to preserve RNA, DNA, and protein integrity.
- Board-certified pathology review and histological validation for every tissue specimen.
- Detailed clinical data, including demographics, diagnosis, treatment history, and outcomes.
- Ethical and regulatory compliance with HIPAA, GDPR, and GCP standards.
- Preservation formats tailored for downstream applications.
Available Specimen Types and Formats
Our biobank services provide access to a broad inventory of human biospecimens from diverse cancer indications and healthy donors, preserved with validated workflows and available in multiple formats to meet specific research goals. Each sample is paired with extensive clinical metadata—diagnosis, stage, treatment history, and outcome data—supporting seamless integration into advanced multiomics studies.
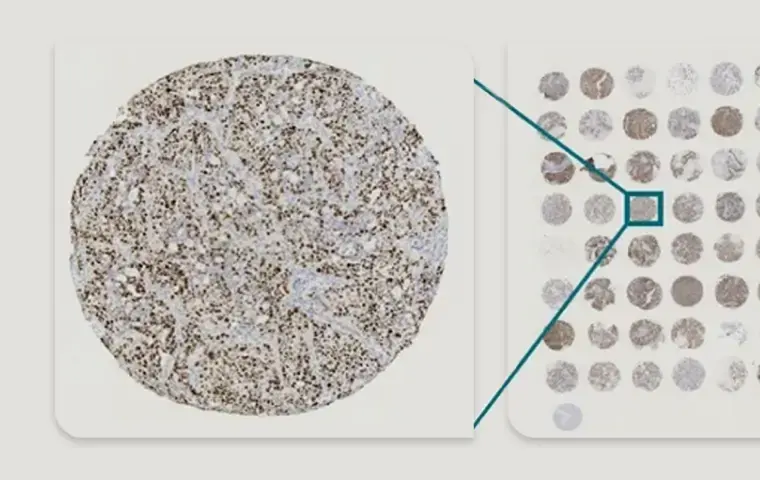
- Fresh-frozen or FFPE tumor tissue
- Matched adjacent normal tissue
- Tumor Tissue Microarray (TMA)
- Available across multiple cancer types (e.g., colorectal, NSCLC, breast)

- Plasma, serum, buffy coat, PBMCs
- Longitudinal collections available for time-course studies
- Suitable for ctDNA, proteomics, cytokine profiling, and more
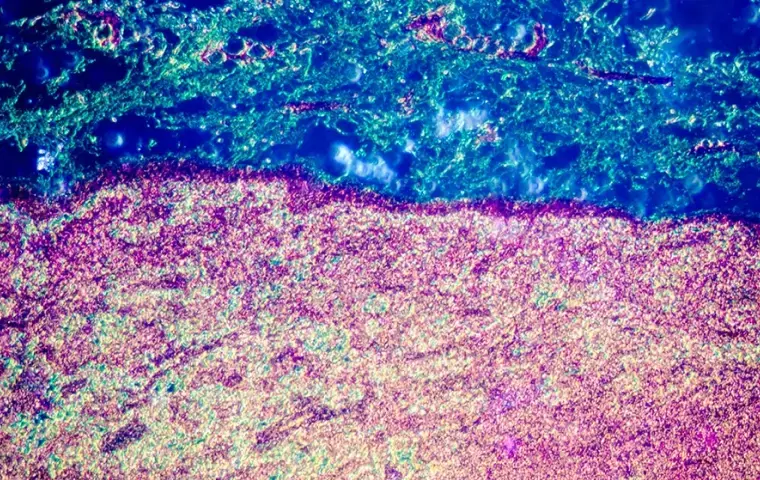
- Prospectively collected fresh tumor and adjacent normal tissue samples based on protocol specifications
- Disease-state specific or treatment-naïve samples upon request
- Tailored cohort design with IRB oversight
Disease Indications and
Molecularly Stratified Cohorts
Our extensive biobank features well-characterized cohorts from treatment-naïve, refractory, and relapsed patient populations—designed to support targeted therapy development, immuno-oncology research, and biomarker validation. This stratification enables researchers to select highly relevant biospecimens for retrospective cohort analysis, predictive biomarker development, and multiomics correlation studies to increase translational relevance and accelerate their insights.
 Our oncology specimen portfolio includes, but is not limited to:
Our oncology specimen portfolio includes, but is not limited to:
- Non-Small Cell Lung Cancer (NSCLC)
- Breast Cancer
- Prostate
- Colorectal Cancer (CRC)
- Liver
- Pancreatic
- Ovarian
- Melanoma and Other Solid Tumors
- Driver mutations (EGFR, KRAS, BRAF, PIK3CA, and others)
- Treatment history (first-line, second-line, and beyond)
- Histopathology and tumor content confirmation
Multiomics vs. Standard Grade Biospecimens
We supply two tiers of human biospecimens: Multiomics Grade for high-precision, data-rich projects, and Standard Grade for scalable, broader research needs. Both are backed by our global sourcing, minimized ischemia times, and extensive clinical annotation.
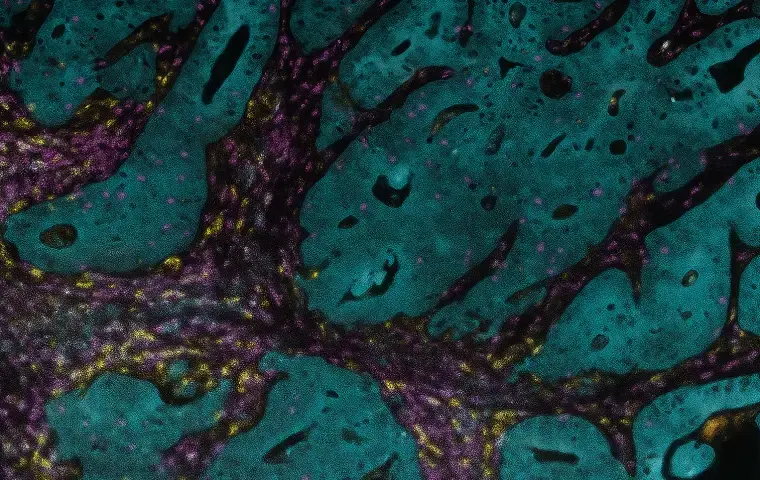
Designed for genomic, transcriptomic, proteomic, and spatial biology applications, our multiomics collection sets the bar for advanced analysis.

Optimized for high-throughput screening, diagnostic validation, and cost-conscious projects, our standard grade maintains stringent quality with practical scale.
Our proprietary Oncology Biobank is a meticulously curated collection of human biomaterials and comprehensively associated clinical data.
- Global Clinical Network
Extensive partnerships ensure diverse and ethically sourced tissue procurement. - Patient Confidentiality
Adherence to stringent international standards for patient data protection. - Rigorous Quality Management System:
- Pathological verification
In-house pathologists perform Hematoxylin and Eosin (H&E) staining for quality control of all tissue samples. - Standardized collection protocols
Uniform procedures across all collection sites ensure inter-sample comparability and reduce pre-analytical variability.
- Pathological verification
Our biospecimen collection and processing workflows are designed to uphold scientific rigor and regulatory compliance at every stage—from patient consent to long-term storage—ensuring sample fidelity for high-confidence research outcomes.
View our quality-related accreditations and certificates.
All samples are processed using standardized preanalytical protocols to minimize variability and maximize molecular integrity for downstream applications, including NGS, qPCR, IHC, spatial transcriptomics, and flow cytometry.
Our Respect for Patients
We have the utmost respect and gratitude for the people who donate their biospecimen. Our mission to ensure the right patient receives the right treatment, at the right time, is underscored by the strength of those who continue to battle cancer. We strive to realize our mission with every day that passes.
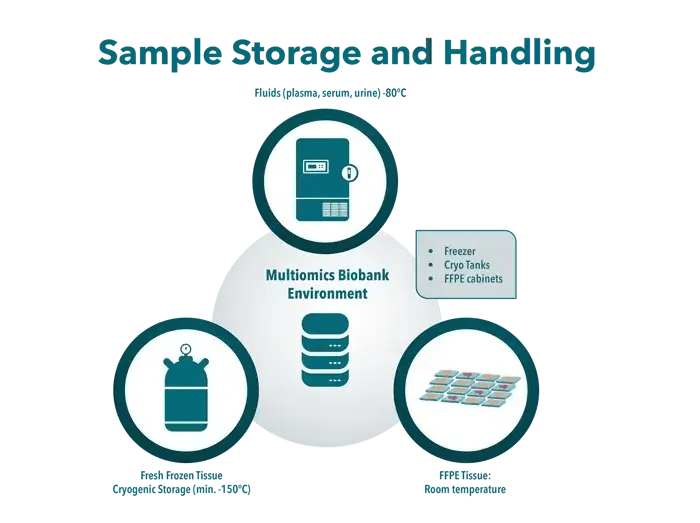
We maintain state-of-the-art biobanking facilities designed for optimal sample preservation and integrity, located in Hamburg and Frederick, comprised of:
- Freezer Systems
Approximately 40 freezers operating at −80∘C for long-term storage of plasma, serum and urine. - Cryo Tanks
Approximately 22 cryo tanks providing cryogenic storage at minimum −150∘C for FF tissue. - FFPE Cabinets
Dedicated storage for FFPE tissue at room temperature, maintaining morphology and molecular integrity. - Integrated Data Management
Robust systems ensure comprehensive tracking and accessibility of all stored biospecimens.
We employ a fully traceable, barcode-based inventory system for real-time sample tracking and full chain-of-custody documentation, capturing:
- Collection timestamps
- Preservation and processing metadata
- Temperature logs during handling and storage
- Pathology verification and tumor content annotation
All biospecimens are accompanied by digitally linked clinical metadata to facilitate cohort selection, stratification, and integrative analysis.
Our biobank services stand apart with ultra-short ischemia times, ensuring unmatched traceability and reliability. Each sample is enriched with over 300 clinical data points, providing an in-depth view of patient outcomes and follow-ups. Comprehensive datasets, including tumor characteristics, therapy response, and biomarker status, empower researchers to drive meaningful insights. This commitment to high-quality, clinically annotated samples is instrumental in achieving replicable results in oncology research.
At Crown Bioscience, we understand that the quality of a biospecimen's pathology is critical for the success of your research. Our team of board-certified pathologists provides expert review and comprehensive analysis for every tissue sample, ensuring clinical relevance and providing validated data for downstream assays.
Our pathological services include:
- H&E Staining
Every tissue biospecimen undergoes Hematoxylin and Eosin (H&E) staining, a foundational step in pathology. This allows for detailed histological review to confirm tumor presence, cellularity, and purity. This pathological verification is crucial for the reliability of any subsequent molecular analysis. - Immunohistochemistry (IHC)
We offer extensive IHC services to detect and localize specific proteins within the tissue. With a catalog of more than 500 pre-validated antibodies, our IHC analysis provides key insights into target expression, biomarker localization, and disease mechanisms, supporting target validation and patient stratification. - Multiplex Immunofluorescence (mIF)
For more complex studies, our mIF services allow for the simultaneous detection of multiple biomarkers on a single tissue section. This advanced technique provides a rich, spatial understanding of protein interactions and cellular phenotypes within the tumor microenvironment, which is essential for immuno-oncology and spatial biology research.
The future of oncology research lies in integrating data from multiple biological layers to create a holistic view of disease. Crown Bioscience’s biospecimens are the ideal starting material for multiomics research, providing the high-quality foundation needed to integrate data from genomics, transcriptomics, and proteomics.
We support multiomics research with:
- Preserved Molecular Integrity
Our samples are collected with a minimal cold ischemia time, ensuring the integrity of DNA, RNA, and protein. This is paramount for accurate genomic sequencing, RNA expression profiling, and protein analysis. - Integrated Clinical Data
Each biospecimen is linked to a rich clinical data profile, including patient demographics, treatment history, and pathological findings. This comprehensive dataset allows for the correlation of multiomics data with real-world patient outcomes, enabling the discovery of clinically meaningful biomarkers. - End-to-End Support
We provide the expertise and infrastructure to support your entire multiomics workflow, from high-quality sample supply to bioinformatics and data analysis. This positions Crown Bioscience as a trusted partner in advancing precision oncology and accelerating drug development.
Pathological Services and Multiomics Solutions
Quickly compare and understand our different services and key features for your applications.
Delivering Premium Clinical Biospecimens Globally
Crown Bioscience provides access to an unparalleled resource for oncology research through its global clinical network. Connecting with top-tier oncology centers across Europe, the USA, and Asia, our network ensures the consistent collection of high-quality, highly annotated human biospecimens. This foundation is critical for researchers investigating tumor evolution, dissecting complex treatment responses, and discovering novel biomarkers.
By seamlessly integrating clinical expertise with advanced biobanking protocols, our network directly empowers the next generation of precision oncology research, accelerating breakthroughs in cancer diagnostics and therapeutics.
Frequently Asked Questions
Fresh-frozen tissue is considered the "gold standard" for applications requiring high-quality nucleic acids (DNA/RNA) and proteins, making it ideal for genomics and proteomics. FFPE tissue is a stable, long-term storage format that is excellent for histology, immunohistochemistry (IHC), and retrospective studies. Crown Bioscience offers both, ensuring you have the right sample type for your specific research needs.
Yes, we offer custom, prospective collections to meet unique research criteria, including specific disease indications, treatment histories, and patient demographics. Our global clinical network allows us to source the precise samples you need for your project.
We use a fully traceable, barcode-based inventory system and have established, validated cold-chain logistics to ensure sample integrity from our biobank to your lab. We manage all shipping and regulatory documentation to ensure a seamless delivery process.
We support the entire biomarker discovery workflow, from initial target identification and validation using our extensive biobank to providing the high-quality, characterized samples needed for clinical validation. Our samples are suitable for multiomics profiling (genomics, transcriptomics, proteomics) to accelerate your discovery programs.
Biospecimens are critical at every stage of drug development. They are used in the early discovery phase to identify novel targets, in preclinical studies to assess drug safety and efficacy, and in clinical trials to stratify patients and monitor treatment response. The quality and characterization of these samples can directly impact the success of a drug program.
Yes. We offer fully traceable samples with robust quality control, making them ideal for the biomarker validation process. Our samples can be used to confirm that your biomarker is reliable, reproducible, and accurate in predicting a specific clinical outcome.
Our biospecimens, paired with rich clinical and pathological data, allow researchers to identify and validate predictive biomarkers that can be used to select the right patients for a clinical trial and guide the development of targeted
Powering Your Next Breakthrough
Ready to accelerate your oncology research with high-quality human biospecimens? We are your trusted partner, providing meticulously characterized samples essential for drug discovery, biomarker validation, and precision oncology studies. Get in touch with us today.

