Biofluid Testing
Hematology analysis, bio fluid chemistry, and biomarker services
Our non-GLP biofluid tests provide robust, high-quality data for early-stage preclinical drug development. These services provide a rapid, cost-effective way to analyze blood and urine samples as a standalone service, or supplementary to in vivo studies.
Analytes listed in the following sections are subject to change. Please contact your local business development specialist for our current offerings. Additional assays are available with a validation lead time of approximately 2-3 weeks.
Blood and Urine Chemistry Analysis
Gain valuable information about drug efficacy, candidate selection, MoA, and enzymatic activity using a comprehensive portfolio of clinical chemistry assays developed on the Beckman Coulter® AU480 Chemistry Analyzer and Sysmex BX-3010 Automatic Chemistry Analyzer.
| Alanine aminotransferase (ALT) | Hemoglobin A1C (HBA1c) |
| Aspartate aminotransferase (AST) | Homocysteine |
| Albumin (ALB) | Ion-specific electrode (ISE: Na, K, CL) |
| Alkaline Phosphatase (ALP) | Iron |
| Beta-2-Microglobulin (B2M) | Lactate dehydrogenase (LDH) |
| Bicarbonate (CO2) | LDL Cholesterol |
| Calcium | Lipemia, Icterus, Hemolysis (LIH) |
| Cholesterol | Lipase |
| C-Reactive Protein (CRP) | Magnesium |
| Creatine Kinase | Urine Total Protein (MTP) |
| Creatinine | Phosphorus |
| CYSC | Total Bilirubin |
| Direct Bilirubin | Triglyceride |
| GGT (Gamma-Glutamyltransferase) | Urea Nitrogen (BUN) |
| Glucose | Uric Acid |
| HDL Cholesterol | Urine Albumin |
Complete Blood Count (CBC)
Platform:
- Element HT5 Veterinary Hematology Analyzer
- VETSCAN ® HM5 Hematology Analyzer
| White Blood Cell Count (WBC) |
| Lymphocytes (LYM) |
| Monocytes (MON) |
| Neutrophils (NEU) |
| Percentage of White Blood Cells (WBC%) |
| Percentage of Lymphocytes (LYM%) |
| Percentage of Monocytes (MON%) |
| Percentage of Neutrophils (NEU%) |
| Red Blood Cell Count (RBC) |
| Hematocrit (HCT) |
| Mean Corpuscular Volume (MCV) |
| Red Cell Distribution Width (coefficient of variation) (RDWc) |
| Red Cell Distribution Width (standard deviation) (RDWs) |
| Hemoglobin (HGB) |
| Mean Corpuscular Hemoglobin (MCH) |
| Mean Corpuscular Hemoglobin Concentration (MCHC) |
| Platelet Count (PLT) |
| Mean Platelet Volume (MPV) |
| Platelet Crit (PCT) |
| Platelet Distribution Width (coefficient of variation) (PDWc) |
| Platelet Distribution Width (standard deviation) (PDWs) |
Biomarker Analysis Services
Translational biomarkers are used throughout drug development to help elucidate underlying pathophysiology, understand an agents’ mechanism of action, measure efficacy, and stratify patient populations. We provide a panel of routinely performed immunoassay services to inform decision-making in drug development, as well as developing, validating, and performing custom assays.
Meso Scale Discovery® (MSD) Biomarker Assays
Run your samples on MSD’s electrochemiluminescence technology platform. Using the Sector S 600, biomarkers can be customized into a V-PLEX or U-PLEX plate with up to 10 biomarkers of your choice.
| Rodent Assays | Human and Nonhuman Primate Assays (NHP Valided Kits) |
|---|---|
| Mouse Adiponectin | 4-Spot Prototype Human Insulin, C-Peptide for NHP |
| Mouse IL-1b | Human/NHP Glucagon |
| Mouse IL-2 | Human/NHP Adiponectin |
| Mouse IL-4 | Human/NHP Total GIP |
| Mouse IL-5 | Human/NHP Insulin |
| Mouse IL-6 | NHP Total GLP-1 (ver. 2 Kit) |
| Mouse IL-10 | |
| Mouse Leptin | |
| Mouse MIP-3a | |
| Mouse Pro-inflammatory 7plex, tissue culture | |
| Mouse Pro-inflammatory Panel 1 (IFN-ץ, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO, IL-10, IL-12p70 and TNF-α) |
|
| Mouse TGFB1 | |
| Mouse TNF-a | |
| Mouse/Rat GLP-1 (7-36 amide) | |
| Mouse/Rat Glucagon | |
| Mouse/Rat Insulin | |
| Mouse/Rat Insulin/Glucagon | |
| Mouse/Rat Total Active GLP1, Insulin, Glucagon | |
| Mouse/Rat Total GLP-1 | |
| Rat BNP | |
| Rat IL-1a | |
| Rat IL-1b | |
| Rat IL-4 | |
| Rat IL-5 | |
| Rat IL-6 | |
| Rat IL-13 | |
| Rat Kidney Injury Panel 1 (Urine Albumin, KIM-1, NGAL/LCN2, Osteopontin) |
|
| Rat MCP-1 | |
| Rat Pro-inflammatory Panel 2 (IFN-γ, IL-10, IL-13, IL-1β, IL-4, IL-5, IL-6, KC/GRO, TNF-α) |
|
| Rat TNF-a |
Colorimetric ELISA Biomarker Assays
Evaluate biomarkers on our standard, colorimetric, enzyme-based ELISA platform.
| Rodent Assays | Human and NHP Assays |
|---|---|
| Mouse Hydroxyproline | Human Cortisol |
| Mouse Urine Albumin | Human IGFBP-1 |
| Mouse/Rat Corticosterone | Human STELLUX Chemi Insulin |
| Mouse/Rat/Human Free Glycerol | |
| Rat Alpha-Fetoprotein | |
| Rat Fecal Corticosterone Cayman | |
| Rat Growth Hormone | |
| Rat TGFB1 | |
| Rat Urine Albumin | |
| Rodent STELLUX Chemi Insulin |
Service Details
Turn Around Time: 5-7 business days (clinical chemistry), 7-10 business days (MSD & ELISA) Data Delivery Format: Excel File, unless otherwise requested
Assay Validation
To ensure you receive reliable data, we perform rigorous quality control and assay qualification procedures to ensure precision and accuracy. Our high quality results enable you to make preclinical go/no-go decisions with confidence.
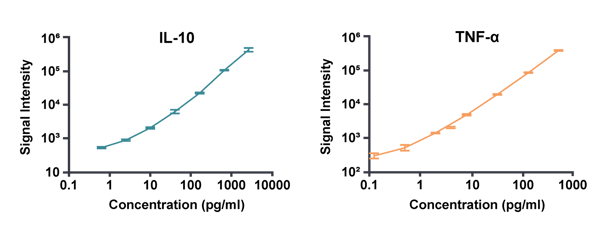 Crown Bioscience's custom-fit, 7-point standard curves used to calculate accurate and precise concentrations for IL-10 and TNF-α.
Crown Bioscience's custom-fit, 7-point standard curves used to calculate accurate and precise concentrations for IL-10 and TNF-α.
Partner with Us
At Crown Bioscience, we are dedicated to advancing your research through cutting-edge technologies like Visium Spatial Transcriptomics. Our expertise in spatial transcriptomics, combined with comprehensive data analysis and tailored support, helps you unlock meaningful, actionable insights directly from tissue samples. Whether you’re investigating tumor microenvironments, tissue heterogeneity, or complex biological systems, our solutions empower you to make groundbreaking discoveries with unparalleled precision and clarity. Connect with us today to elevate your spatial biology research.
