- Services
- Therapeutic Areas
- Model Systems
- In Vitro
- In Vivo
- Technologies
- Service Type
- About Us
- Our Science
- Start Your Study Now
Xuesong Huang, Gang Chen*, Lei Zheng, Annie An, Jean-Pierre Wery, Jay Liu*, Xin Dong*, Qian Shi, and Davy Xuesong Ouyang
*Nanjing Galaxy Biopharmaceutical Co. Ltd., Nanjing, China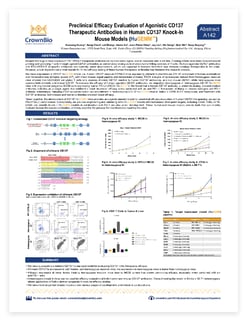
PD-1/PD-L1 checkpoint inhibitors have achieved huge clinical success (and drug approval) across a range of cancer types. However, overall response rates to these agents remains low.
Efforts are now focused on combination strategies which could improve T cell tumor killing activities. For example, agonist CD137 antibodies could be used in combination with anti-PD-1/PD-L1 agents to both prime and activate T cells.
Research into these antibodies is being hindered by a lack of preclinical animal models for the in vivo assessment of human-specific therapeutics. CrownBio has developed a range of humanized drug target immunocompetent models, including a CD137 model, to enable novel immunotherapy and combination evaluation.
Your privacy is important to us.
We'll never share your information.
© 2024 Crown Bioscience. All Rights Reserved.


© 2024 Crown Bioscience. All Rights Reserved. Privacy Policy
2021-10-29
2021-10-29
landing_page
PDX/Databases