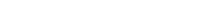Non-GLP Exploratory Toxicology Studies in NHPs
Evaluate the physiological effects and safety of your immunotherapies with our comprehensive non-GLP NHP platform. Our models provide you with quick, cost-effective, and robust data to guide future GLP work and clinical trials.
NHPs are the only clinically-relevant model for assessing immune-related toxicity, allowing preclinical evaluation of potentially fatal clinical toxicities e.g. cytokine storm. NHPs have a high homology to humans, fully intact immune system, and cross-reactivity to antibodies which are not recapitulated in rodents or other larger animal models.
Advantages of our Non-GLP NHP Platform
- Save time and simplify logistics with I/O NHP toxicology and murine studies run at the same facility. Seamlessly move from mouse I/O work to NHP toxicology
- Reduce development time and improve cost effectiveness with onsite pathology/histopathology services, including IHC, ICC, and IF
- Rapid study initiation for fast turnaround of robust results - studies can be started within 4 weeks (dependent on test article availability), with study duration optimized to fit client and project needs
- Cost-effective pathway to guide your GLP studies – our exploratory non-GLP toxicology screen typically runs at a fraction of the cost of a GLP study
Comprehensive Non-GLP Analysis
Assess the immuno-safety of a range of I/O agents including mAbs, CAR-T cell therapies, ADCs, vaccines, small molecules, cytokines, and oncolytic viruses using:
- Healthy immunocompetent cynomolgus macaques
- A range of common administration routes
- Intravenous injection or infusion
- Intramuscular injection
- Subcutaneous injection
- Oral routes – via diet or gavage
- Perform FACS and immunoprofiling of common immune cell markers including cytokines, CD markers, immune checkpoint targets with fast turnaround on additional marker validation
- Analyze serum markers using industry gold standards: Beckman Coulter AU480 clinical chemistry analyzer, Luminex, MesoScale Discovery single and multi-plex systems
- Perform clinical observations to identify potential adverse effects related to cytokine storm/CRS
- Changes in heart rate and/or body temperature
- Facial redness or swelling
- Vomiting, diarrhea, tremor, lethargy, low blood pressure, difficulty breathing
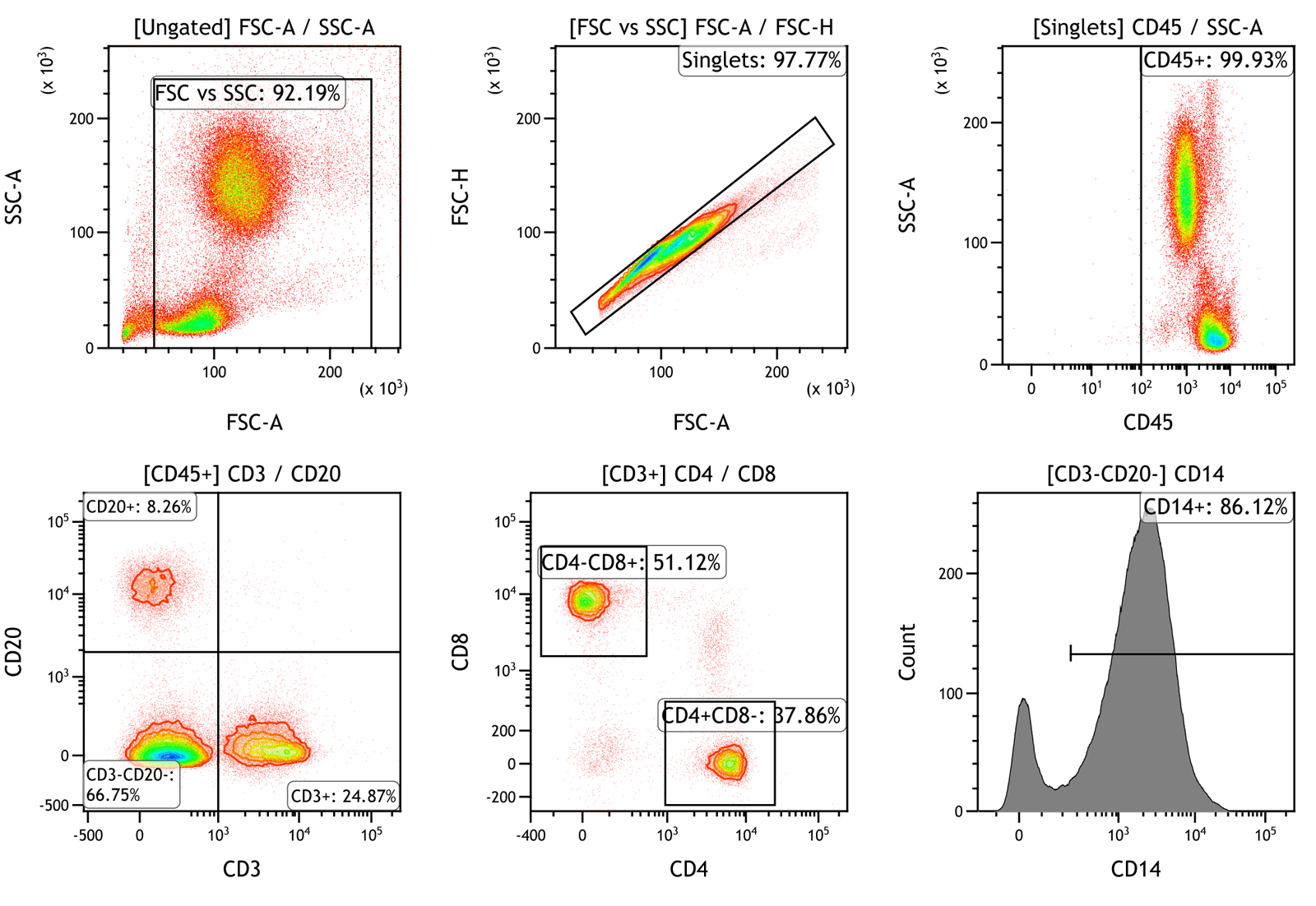
Figure 1: Representative NHP FACS profiling data
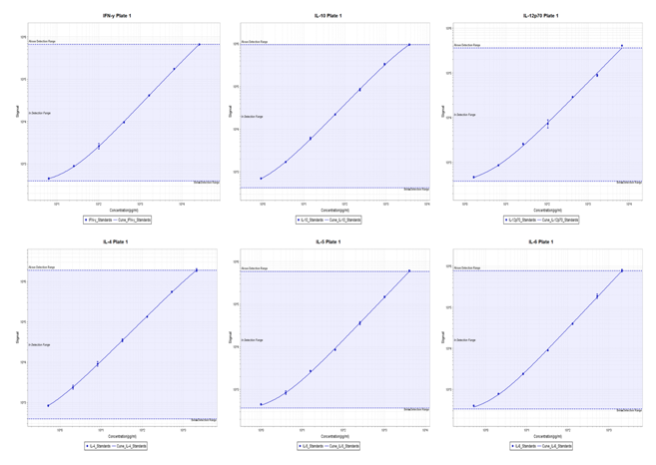
Figure 2: Representative cytokine and chemokine PLEX data
|
Marker
|
Immune Cell Population
|
|---|---|
| CD45 | Total leukocytes |
| CD3 | Total T cells |
| CD4 | CD4+ T helper cells |
| CD8 | CD8+ Cytotoxic T cells |
| CD44/CD62L | Naive, memory and effector T cells |
| CD69/CD44/OX40/CD25 etc | Activation markers |
| CD4+CD25+FoxP3+ | Regulatory T cells |
| CD11b+IA/IElow/- | G-MDSC and M-MDSC |
| Ly6c/Ly6g | Macrophages |
| CD11b+ F4/80 | M1 and M2 Macrophages |
| IA/IE/CD11c/CD206 | NK cells |
| CD3-CD335+ | NKT cells |
| CD3-CD335+ | B cells |
| CD19 | Cytokines |
| TNF-a/IFN-r/IL-7/IL-3 etc | Checkpoint inhibitors |
| Granzyme B etc | Commonly requested markers |
| KI67/Brd U/PNCA et | Proliferation |
| Live/Dead (fixable) | Live/Dead |
| CD45 | Total leukocytes |
| CD3 | Total T cells |
Table 1: Target panel for NHP profiling