- Services
- Therapeutic Areas
- Model Systems
- In Vitro
- In Vivo
- Technologies
- Service Type
- About Us
- Our Science
- Start Your Study Now
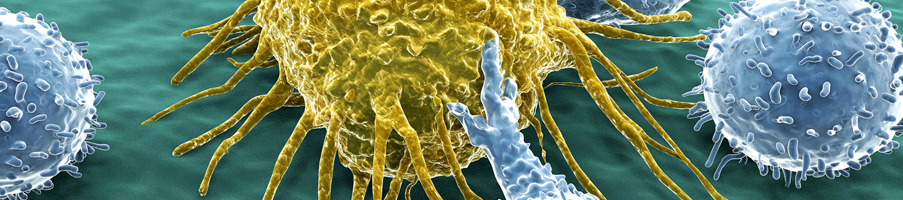
Immuno-oncology (I/O) being such a highly diverse therapeutic area requiring specialist knowledge to progress in the field. The development of new immunotherapies still faces many challenges. Crown Bioscience have gained unrivalled preclinical and translational I/O experience through our partnerships with the world's leading I/O companies.
Leverage our I/O in vitro and ex vivo services to quickly identify the right target for your immunotherapy. We offer a wide range of assays and platforms for in vitro testing of I/O agents, including core immunophenotyping, functional assays, and technologies for the regulation of the tumor microenvironment, allowing you to move with confidence to the next phase of drug development.
We understand that the complexity of I/O requires niche preclinical models for the most effective and translatable results. With that, we’ve developed a variety of validated in vivo models, specifically designed to answer a wide range of research questions.
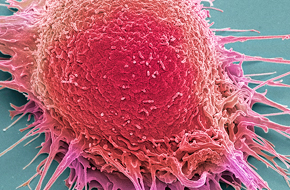
Make key decisions in a timely and cost-effective manner about the progression of your preclinical development program with cell line-derived xenograft (CDX) models.
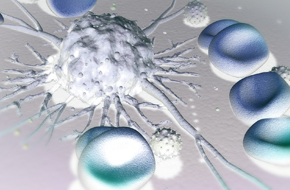
Discover homografts derived from immortalized mouse cancer cell lines with fully competent immunity for assessing single agent and combination immunotherapies.
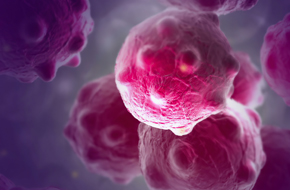
Harness the human immune system against human tumors with human hematopoietic stem cell (HSC) engraftment of patient-derived xenograft models.
Explore a simple alternative to the full stem cell reconstitution approach that allows you to evaluate your immunotherapy within a human tumor microenvironment.
We have expertise in applying these models and assays with range of immunotherapeutic's such as checkpoint inhibitors, vaccines, oncolytic viruses, microbiome modulators, cellular therapies (CAR-T), bi-specific T Cell engagers and various immune modulators.
Tell us about your needs
© 2024 Crown Bioscience. All Rights Reserved.


© 2024 Crown Bioscience. All Rights Reserved. Privacy Policy
2024-01-29
2021-10-23
site_page