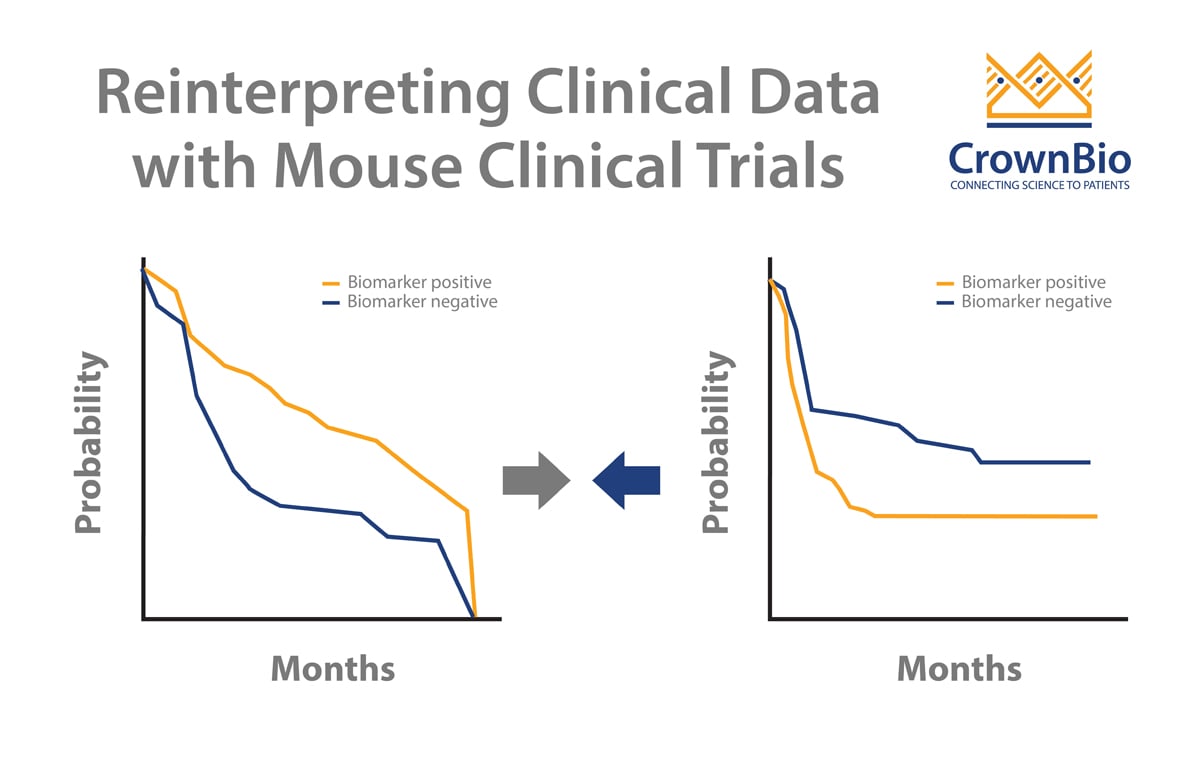
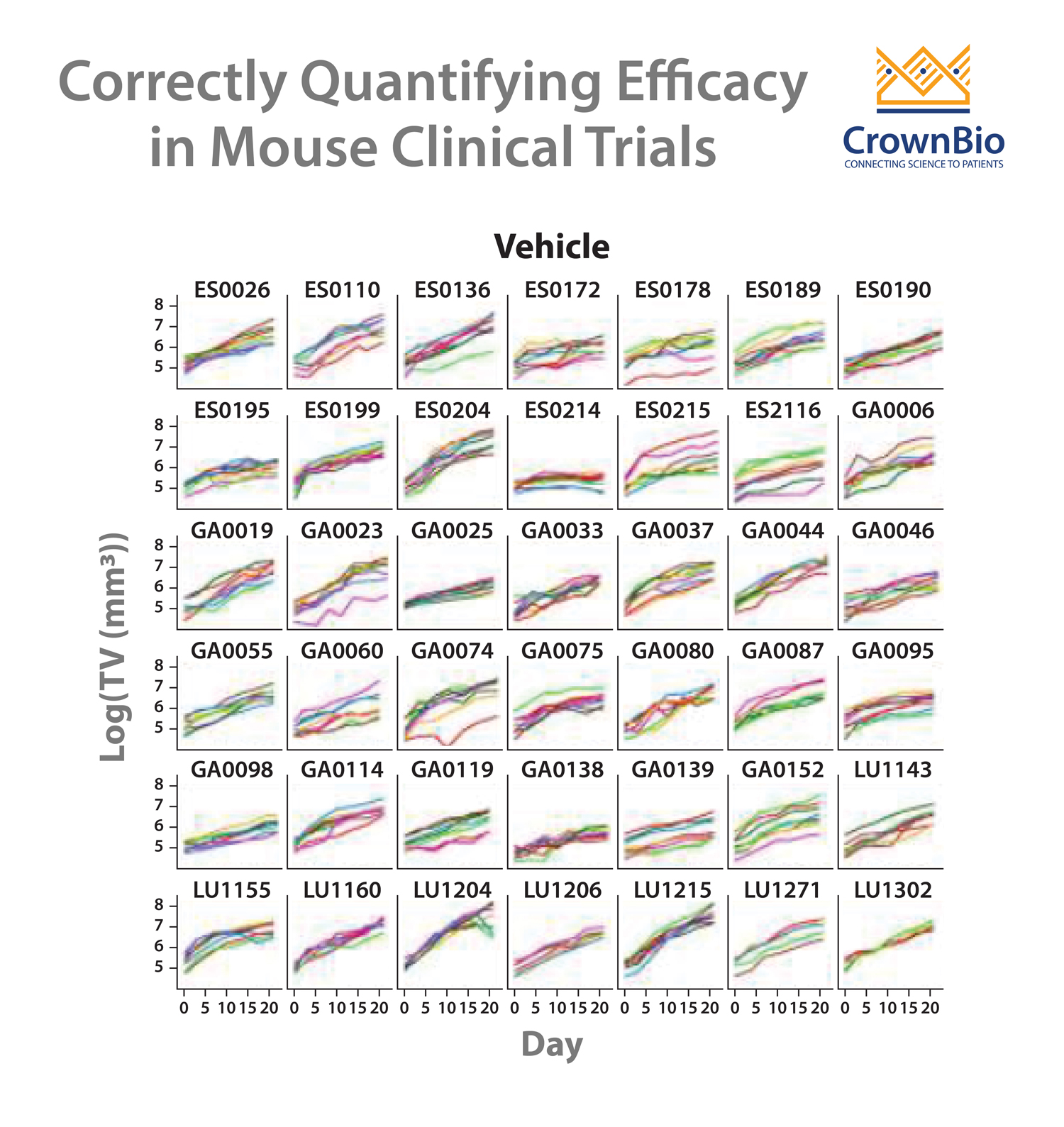
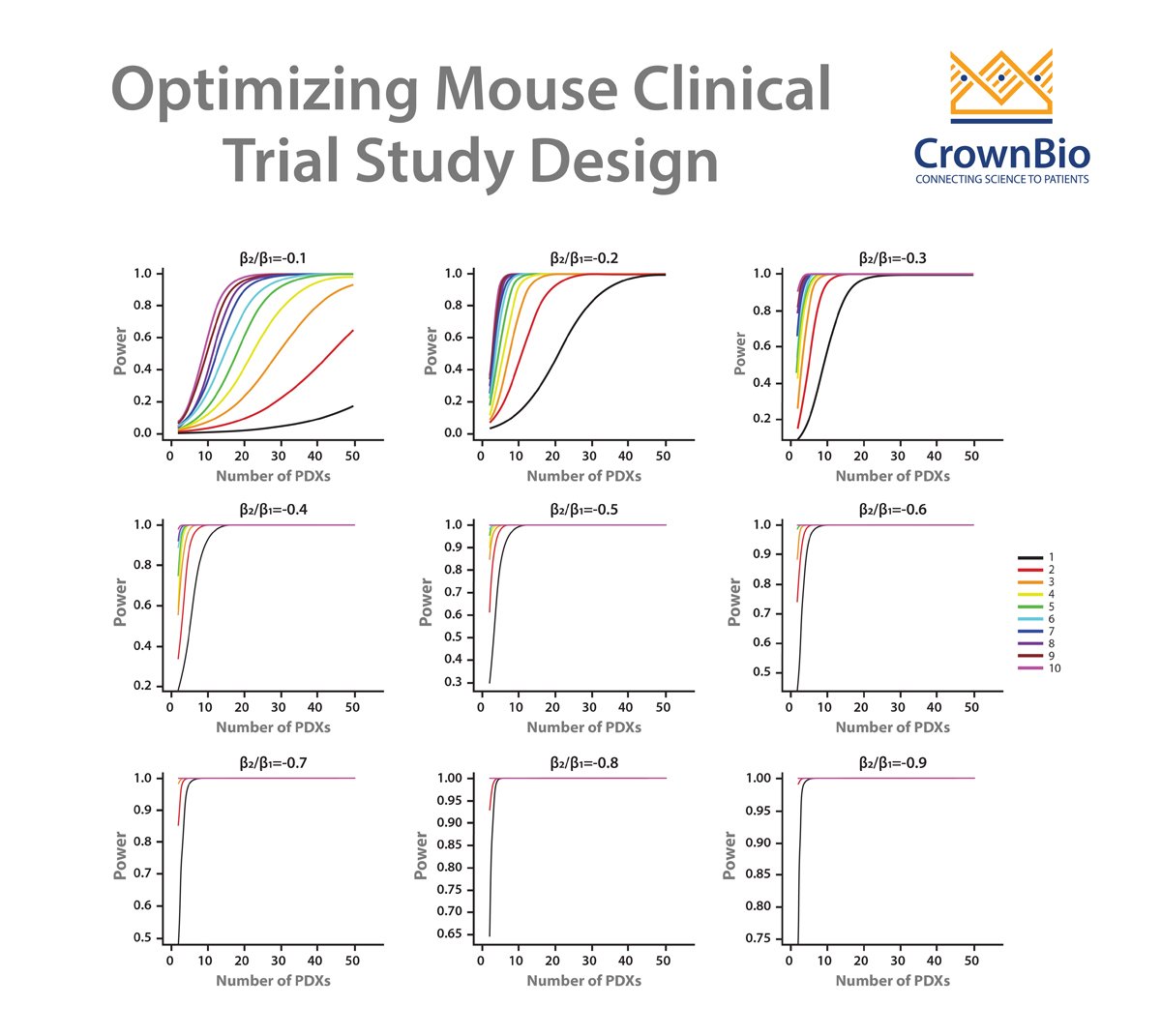
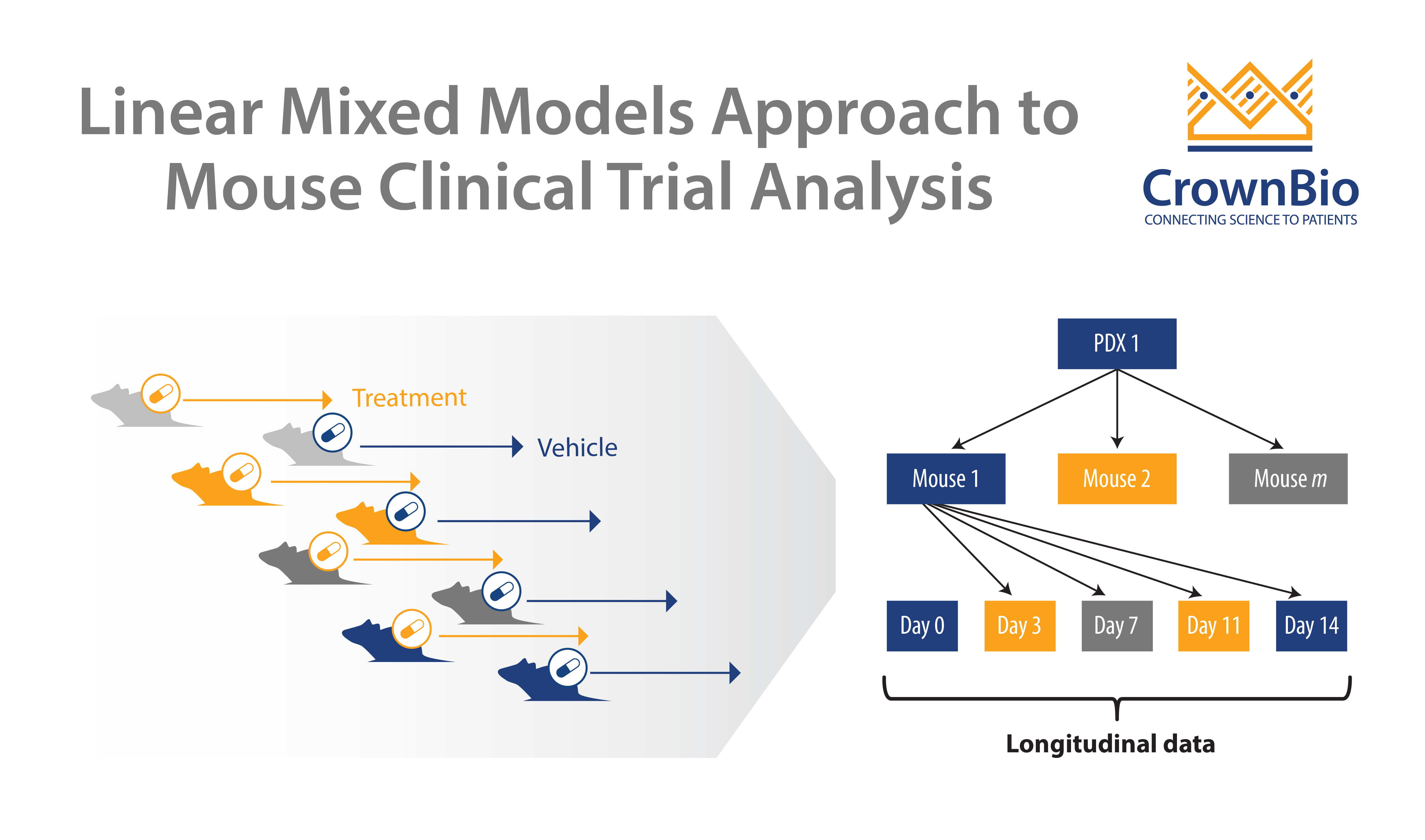
Patient participation in oncology clinical trials is very low due to patient concerns including worries over experimental drug effectiveness and uncertainty around joining clinical trials versus using a standard therapy. New methods are needed to encourage higher enrolment, one of which is ‘window of opportunity trials’ which use gaps in early standard of care treatment regimens to trial novel anticancer agents.
Data collected from human clinical trials are the critical part of approval of new drugs in all therapeutic areas including oncology. Unfortunately, the number of adults who take part in trials for novel oncology agents is very low. Statistics from the UK 10 years ago showed that around 11% of people with newly diagnosed cancer participated in clinical trials. More recent data from the US show that only 3% of adults with cancer take part in trials, with 40% of studies (including three out of five Phase III trials) failing to achieve minimum patient enrolment.
There are many valid reasons why cancer patients don’t enroll in trials including patient preference for a specific treatment, worries about uncertainty of trials or receiving a placebo, concerns that an experimental drug might not be the best option compared with tried and tested standard of care agents, potential side effects, and physician influence. Encouraging more patients to join trials will speed up agent approval and provide more reliable data for the drugs being tested, but requires one or more of the patients doubts to be resolved. One method helping to overcome patient concerns is window of opportunity trials – which involves testing new agents in gaps between standard cancer treatments, which hopefully encourages more patients to take part.
Window of opportunity trials can take a variety of inter-related forms, all making use of the small gaps in standard cancer therapy. Many of these trials take advantage of gaining data from untreated patients rather than the heavily pretreated population often recruited to late stage trials. For example, in breast cancer a novel study drug can be given to patients between a diagnostic breast biopsy and planned surgical resection. It is hoped that this type of study can improve understanding of an agent's biologic effect and potential target population early in its development without disturbing the standard treatment pattern of a patient. Trials of this type provide tumor tissue from before and after the new therapy treatment (in chemo- and radiotherapy naïve patients) for biomarker analyses of response and potentially of resistance development.
A second window of opportunity method sees patients slightly delaying their standard of care regimen by a predefined time, to receive a novel treatment. This again allows data to be gathered on the drug that is not skewed by previous or simultaneous treatment that could modify the genetics of the tumor and cause resistance to the novel drug. For patients wanting to start treatment immediately, window of opportunity trials also utilize gaps in prescribed chemotherapy to trial new agents, to see if the new drug offers any additional improvements over the standard treatment.
These trials hopefully remove some patient issues around uncertainty of trials, patient preference for a specific treatment (which they can still receive), and concerns that a novel agent may not provide the best results, as they still receive a standard of care treatment. Data from early stage cancer patients in these trials is essential to really understand how a drug is working, any mechanisms of action of resistance, and to better understand responder populations for later stage trials. Improved enrolment levels should also hopefully see a quicker route to market for many lifesaving new therapies.
Crown Bioscience are happy to see any new techniques which improve patient recruitment and which can provide more translational resources such as tumor tissue for biomarker analysis. We help to identify biomarkers and responder populations at the preclinical level through our Translational Oncology Platforms – Crown Bioscience utilize the world’s largest commercial collection of >1,100 genomically characterized patient-derived xenograft models (HuPrime®; large enough to be truly reflective of the patient population) to run HuTrials™, preclinical Phase-II like, human surrogate trials to evaluate your oncology agents. We use our HuMark™ Translation Platform which spans a range of experiments and analyses from tissue collection to human surrogate trial data analysis to predict biomarkers of response to your agent, and to help to validate antitumor effects against predicted human targets.
Contact us at busdev@crownbio.com to talk to our experts on how we can further your translational oncology needs through our patient-derived xenograft models and oncology resources today.